Array of electrodes for measuring brain neuronal activity in vivo
Published in Bioengineering & Biotechnology
Understanding the coordinated activity of neural cells is a task raided by technical limitations with the main restriction being resolution. To detect the electrical stimulus of individual neurons and synapses, contrast needs to be generated in the micrometre range, and accessed across the different brain layers. However most microscopic modalities are restricted to detecting signal from the superficial cortical layers, whereas other in vivo methods, such as electroencephalography, do not have single-cell resolution. Reporting in Nature, Timothy Harris and co-authors describe a silicon probe, named Neuropixels, which is thin enough to be implanted in the mouse brain and capable of processing signal across 384 switchable channels from 960 detectors. This implantable chip is 10-mm long, allowing its positioning to access and gather information across an axial slice of the rodent brain, for example simultaneously across the visual cortex, the hippocampal subfields and the thalamus (Figure 1). The chip is only 20 micrometres in cross-section and 70 micrometres wide and can be placed to record neural activity in awake, freely moving animals. The chip's 960 densely packed recording sites fabricated with low impedance titanium nitride (TiN), each measuring 12 x 12 micrometres, can detect, amplify, multiplex and digitize electrical signal originating from hundreds of individual neurons.
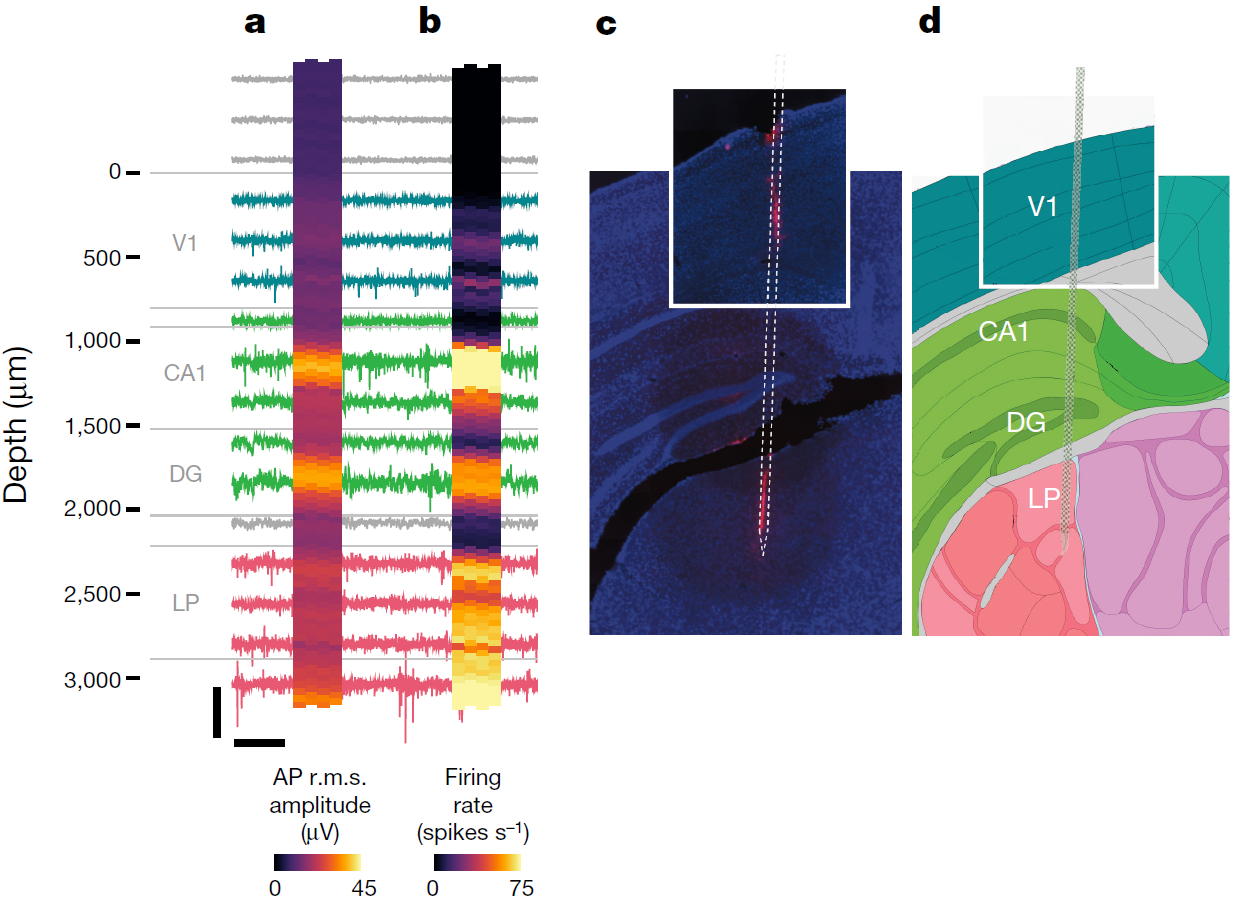
Figure 1: Recording from large neuronal populations with a single probe in an awake head-fixed mouse. Signals were acquired from a Neuropixels probe inserted through primary visual cortex (V1), CA1 and dentate gyrus (DG) regions of the hippocampus, and the lateral posterior (LP) nucleus of the thalamus. Approximate structure boundaries are shown in grey next to the probe depth scale. For the heat maps, each square represents a single site. a, root-mean squared (r.m.s.) amplitude of the action potential (AP) band signal for 1-s intervals, averaged over 10 intervals. b, Firing rate measured from AP band crossings of a − 50 μ V threshold, in a 10-s interval. c, Histological reconstruction of the probe track with 4′ ,6-diamidino-2-phenylindole (DAPI; blue) and DiI (red) staining. d, Corresponding images 81 and 82 from the Allen Mouse Brain Atlas. Image and caption adapted from Jun G.G. et al., Nature, (2017).
The authors ensured the development of the probe minimizes any movement artefacts and is suitable for long-term (over eight weeks) chronic monitoring of the animals, which are fitted with a head mount (for data transfer and amplification) yet are able of moving freely. The implants have been successfully tested in vivo for up to 150 days. For this purpose, the chips were developed to have on-board 10-bit analogue-to-digital converters and a complementary metal–oxide–semiconductor (CMOS) digital microprocessor. The data from each channel is split into two frequency bands, one recording action potentials (0.3-10 kHz) and the other recording local field potentials (0.5-1000 Hz). The signal from each site is then displayed as a pixel and, using neural activity characteristics such as the simultaneous firing or signal amplitude within specified frequency ranges, the data can be used to generate images with structural boundaries. As an example, a recording of 206 neurons with strong association between electrical signal and visual stimuli confirmed, by histopathological analysis, the anatomical identification of brain regions such as the visual cortex and the lateral posterior nucleus of the thalamus. Multiple implants can be also used. In a proof-of-concept experiment, the authors show the ability of one probe to monitor the visual cortex, the hippocampus and the thalamus, whereas a second probe was placed to cover the motor cortex and the striatum covering a total of 741 neurons. The electrophysiological signals are analysed for yield (number of neurons per structure), efficiency (number of neurons detected per site), density and spread, and generate outstanding coverage of neuronal activity in vivo. The implants are also compatible with optogenetic technologies, for which the signal generated from the excitation light can be filtered out of the data.
The main advantages in the design of this device are its small footprint, its sensitivity and the scalability of the low-cost technology. The ability to monitor neuronal electrophysiology with high temporal resolution is expected to provide a new wealth of information with regards to the coordination of neuronal functional coordination.
Highlighted paper:
Jun, J.J. et al., Fully integrated silicon probes for high-density recording of neural activity. Nature, doi:10.1038/nature24636, (2017).
Further reading:
Scholvin, J. et al. Close-packed silicon microelectrodes for scalable spatially oversampled neural recording. IEEE Trans. Biomed. Eng. 63, 120–130, (2016).
Rios, G. et al. Nanofabricated neural probes for dense 3-D recordings of brain activity. Nano Lett. 16, 6857–6862, (2016).
Shobe, J. L. et al. Brain activity mapping at multiple scales with silicon microprobes containing 1,024 electrodes. J. Neurophysiol. 114, 2043–2052, (2015).


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in