Insight into catalytic processes in confined spaces
Published in Electrical & Electronic Engineering

The use of zeolites as catalysts in industry is a rapidly growing global phenomenon due to the fact they can be used in processes that provide 'drop-in' replacements to fossil fuels. The stability of their catalytic performance is perhaps the major challenge for the widespread use of this technology, specifically the fast formation of carbon deposits during operation severely compromises process efficacy both due to this short effective lifespan and the cleaning process required for reactivation.
Despite much research on zeolites over the past 40 years, their reaction mechanisms are complicated and there is still much to be learned. In that regards, spectroscopic techniques such as Raman spectroscopy, particularly when applied under operando conditions (i.e. as the catalysis takes place) can help to understand some of the evolving chemistry within the pores of the material. However, there is a major problem; sample fluorescence that occurs during the measurement process that can often swamp the signal rendering the data at best difficult to interpret but at worse, useless!
However, by applying a technique called Kerr Gate Raman, which was first developed at the CLF in 1999 (P. Matousek et al. Appl. Spectrosc, https://doi.org/10.1366/0003702991945993) our team at University College London and based at the Research Complex at Harwell, in collaboration with the Centre for Molecular Modeling (CMM at Ghent University) and the Central Laser Facility (CLF), made an insightful discovery about industrial catalysts using the CLF’s Ultra Laser. The article can be found in Nature Materials (I. Lezcano-Gonzalez at al. Nat. Mater. (2020). https://doi.org/10.1038/s41563-020-0800-y) and reports new understanding about what really goes on in these industrial catalysts when they deactivate. This discovery could potentially lead to future improvement in catalyst efficiency.
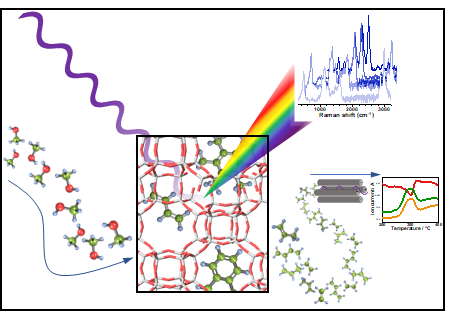
Figure 1. Operando Kerr Gate Raman during the methanol-to-olefin reaction allows for identifying species that lead to catalyst deactivation.
The Raman Kerr Gate: Raman spectroscopy involves detecting light from a laser beam that is scattered from an object; a tiny proportion of this scattered light interacts with molecules in the object causing the scattered light to change colour. This colour change is like a unique “Raman fingerprint” that can be used to identify what molecules in the object are made of.
However, the issue with zeolites is that they emit a large amount of fluorescence during the interaction. This completely overwhelms the tiny Raman signals and makes it practically impossible to detect the useful Raman fingerprint.
Our team used the Ultra facility to apply a Kerr Gate modification to Raman Spectroscopy. This allowed us to take advantage of the different properties of fluorescence and Raman light, where Raman scattering happens instantly while fluorescence happens tens of picoseconds to nanoseconds after interacting with the object. This means that by using a picosecond pulse length laser for the Raman scattering it is possible to “gate out” the unwanted fluorescence after letting the Raman light come through.
The “Gating” in Kerr Gating relies on some very interesting physics to work. An intense laser beam operating in the near infrared triggers a liquid material called carbon disulphide to align its molecules briefly, making it birefringent (meaning that any light passing through changes polarisation). This lasts for about 3-4 picoseconds, after which the molecules return to normal and lose their birefringence.
When the picosecond laser beam hits the object, the Raman light scatters in a picosecond burst with the fluorescence light coming just after. Lenses collect the Raman and fluorescence light and it is then vertically polarised using a polariser before arriving at the Kerr Gate (the carbon disulphide). Due to the gate’s birefringence, the Raman lights polarisation rotates to the horizontal plane. However, by when the fluorescence arrives at the gate, the birefringence has been lost, leaving the fluorescence vertically polarised.
Both types of light then in turn reach a crossed polariser that only allows the horizontally polarised Raman light through and blocks the vertically polarised fluorescence, leaving scientists with a clear Raman signal to read.
Using this technique, the team could see around 100X improvement in the signal quality and discovered something novel about the way the zeolites deactivated.
The science: For years, it was commonly thought that the formation of polycyclic aromatics on the surface of the zeolites was responsible for their deactivation. However, using a combination of Kerr gate Raman Spectroscopy and some insightful computational modelling, we were able to determine that what actually deactivates them is a species known as polyenes, which form before eventually reacting further to form the commonly found polycyclic aromatics.
This was a completely new and unexpected finding. It is with this knowledge the team has proposed a design criteria for zeolites with ideal characteristics in order for them to last far longer.
There are not only financial implications in doing this, but environmental. There are huge energy costs associated with having to reactivate catalysts frequently, so the longer they last for the more energy efficient they are. With this new knowledge, we are planing to continue our investigation into understanding how industrial catalysts work and how we can improve their performance.
Follow the Topic
-
Nature Materials

A monthly multi-disciplinary journal that brings together cutting-edge research across the entire spectrum of materials science and engineering, including applied and fundamental aspects of the synthesis/processing, structure/composition, properties and performance of materials.






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in