Can a Study set a New Standard in Chronic Pain Understanding and Data Capture?
Published in Bioengineering & Biotechnology, Neuroscience, and Research Data

The "Why"
Chronic pain is challenging to study due to its subjective nature and the limitations of current assessment tools. Paper diaries, while easy to use, can lead to errors as they depend on patients’ memories. This is called the “parking lot” effect, where patients may fill out their diary entries in the parking lot or waiting room right before a visit. Since the data is not logged when the episode occurs, the data is susceptible to recall errors and becomes questionable in terms of its accuracy. And if sessions are logged in a compliant manner, there is no way to verify if they were reported at that exact time and date. The transition to electronic diaries (eDiary) has been driven by this need for greater accuracy, compliance, and data security in clinical trials. Regulatory bodies like the FDA have also emphasized the importance of patient-reported outcomes (PRO) and ePRO for supporting clinical claims. Traditional pain studies with devices typically have patients report pain at a baseline visit, and one at some pre-defined timepoint or timepoints in the future. If pain decreases by a certain amount, such as 50% from baseline, a patient is considered to have responded to therapy. This can work for typical neuromodulatory devices that are in the “on” state continuously, such as Spinal Cord Stimulators or Peripheral Nerve Stimulators. However, if a device is used “on-demand” for pain therapy, the typical study designs mentioned would not allow for the appropriate data capture to truly understand its effect on patient pain levels. With these limitations in mind, Neuros Medical created a patented data collection and analysis system that could transform the understanding of chronic pain through the robust data that were captured[1,2]. This ePRO collection and analysis methodology was utilized in the pivotal QUEST-IDE trial to assess the ability of High Frequency Nerve Block (HFNB) delivered through the Altius® High Frequency Stimulation System (HFSS) in reducing chronic post-amputation pain.
The "How"
As the patients in the QUEST Study used Altius HFSS to treat their pain any time of the day or night, their eDiary needed to be equally flexible to capture all these uses. Treatment Report sessions were available to patients whenever they used the device. Patients reported their pain levels in “real-time” at the start of a treatment report session and were prompted by alarms from the eDiary to report their pain at 30-minutes and 120-minutes after treatment. At the end of each day, patients were given alarms to complete a summary pain report. Patients also reported their pain medication use and prosthetic use. Over the course of the entire study, 607 patients reported 1.8 million datapoints across 198,000 reports with high levels of compliance (77%-82% depending on report type). The 170 patients who were included in primary and secondary effectiveness and safety endpoints analyses reported their pain continuously from enrollment to their Month-12 visit. These data were then combined with data captured during study visits to complete our statistical analysis around the QUEST Study Endpoints (Fig. 1).
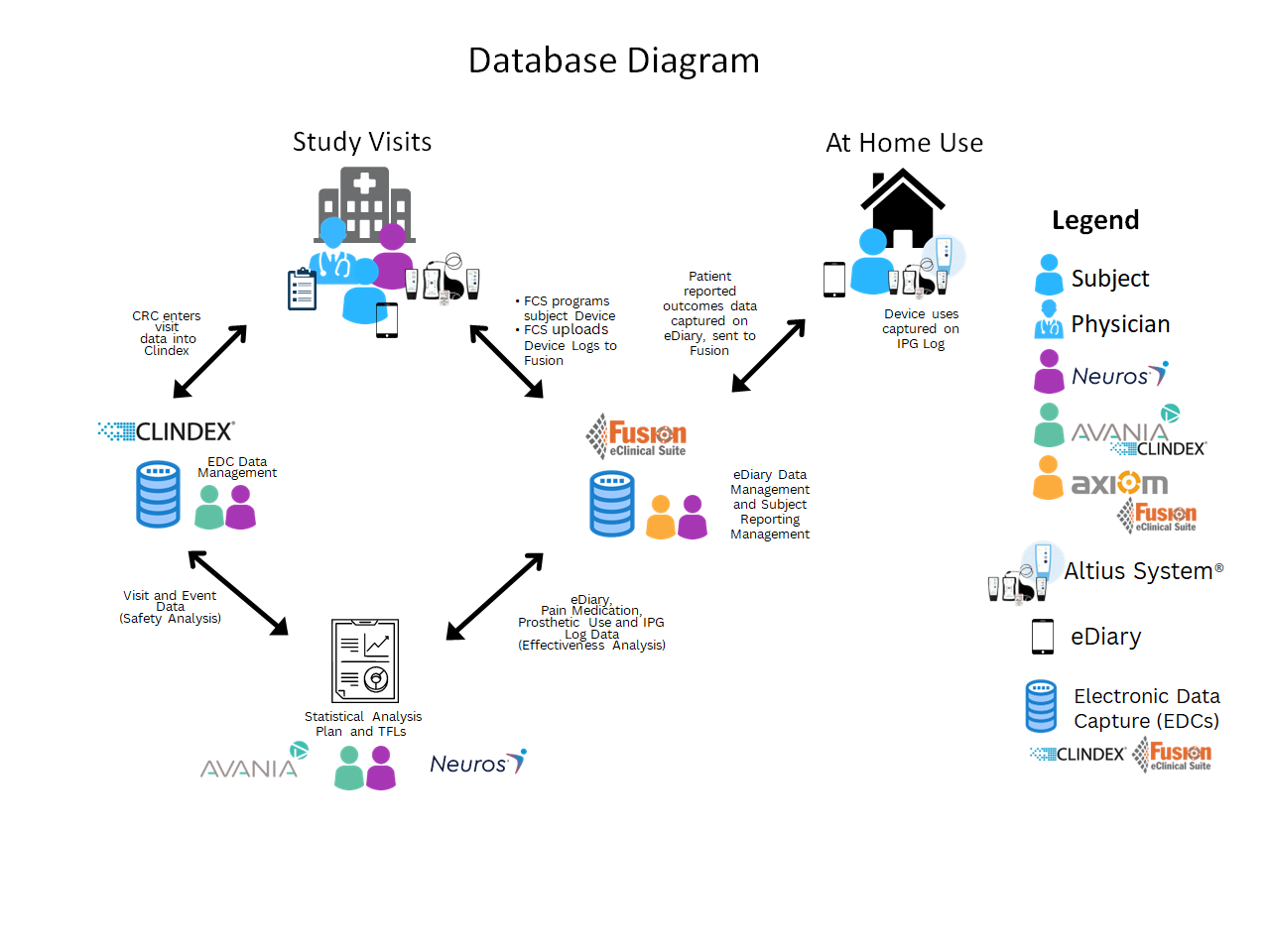
Fig.1: Diagram for QUEST data flows. Subjects report on their eDiary (At Home Use) where reports were submitted to the Fusion database. Fusion also contained electronic copies of IPG logs gathered at programming visits (Study Visits). Within Fusion, IPG sessions were paired with eDiary sessions. Data from these paired session reports were then used for the primary and key secondary endpoint analyses. End-of-Day Reports provided additional medication and prosthetic use information for secondary endpoint and supplemental analyses. Additional data were captured at study visits via case report forms and entered into Clindex EDC. These data were then used to analyze all primary safety endpoints and other event data. Adapted from [1].
The "Future"
Neuros’ patented data collection and analysis system captured the unique capabilities of Altius® to significantly reduce patient pain, decrease opiate usage, and improve quality of life (details published elsewhere) [3]. We also feel the implications of this data system extend beyond the QUEST study, such as:
- Capturing Pain Complexity: Our data system can capture the intricate details of a patient's pain journey, ensuring even subtle changes are recorded.
- Linking Treatment to Progress: By accurately associating treatments with long-term alterations in pain profiles, the system provides a clear picture of impact over time.
- Blueprint for Chronic Pain Clinical Trials: Serving as a model for future research, our system lays the groundwork for conducting large, multi-center, double-blinded, randomized, active-sham controlled trials. These studies, along with their repeated measurements, can pave the way for a new era of pain management research.
We hope this manuscript and study serve to engage the chronic pain community and broader medical and research communities around what is possible as we strive to relieve pain and restore lives.
References
- Iorio, M., Legaspi, R., Syed Shah, N. et al. Learning pains: system design, management, and lessons learned using electronic patient reported outcomes in the QUEST study of chronic post-amputation pain. Discov Health Systems 3, 26 (2024). https://doi.org/10.1007/s44250-024-00091-9
- Iorio M, Syed Shah N. System and method for quantifying qualitative patient-reported data sets (U.S. Patent No. 11,878,172). U.S. Patent and Trademark Office; 2024.https://image-ppubs.uspto.gov/dirsearch-public/print/downloadPdf/11878172
- Kapural L, Melton J, Kim B, Mehta P, Sigdel A, Bautista A, Petersen EA, Slavin KV, Eidt J, Wu J, Elshihabi S, Schwalb JM, Garrett HE Jr, Veizi E, Barolat G, Rajani RR, Rhee PC, Guirguis M, Mekhail N. Primary 3-Month Outcomes of a Double-Blind Randomized Prospective Study (The QUEST Study) Assessing Effectiveness and Safety of Novel High-Frequency Electric Nerve Block System for Treatment of Post-Amputation Pain. J Pain Res. 2024;17:2001-2014
https://doi.org/10.2147/JPR.S463727
Follow the Topic
-
Discover Health Systems

This journal takes a multidisciplinary approach to address systems-level research and discussions relating to health systems, services and informatics, reflecting health outcomes, including from business and health policy perspectives.
Your space to connect: The Psychedelics Hub
A new Communities’ space to connect, collaborate, and explore research on Psychotherapy, Clinical Psychology, and Neuroscience!
Continue reading announcementRelated Collections
With Collections, you can get published faster and increase your visibility.
Building Resilient Health Systems and Outbreak Preparedness in Africa: Policy, Governance, and Technological Innovation
Achieving timely prevention, detection, and response to infectious disease threats, while maintaining equitable access to essential services—depends on robust, well-governed health systems. In Africa’s diverse contexts, strengthening these systems requires integrated policy frameworks, adaptive management practices, and innovative technologies that address gaps in surveillance, workforce capacity, supply chains, and community engagement.
This Article Collection examines multidisciplinary strategies to enhance health system resilience and epidemic preparedness across the continent. We focus on:
- Policy and Governance: Crafting adaptive national and subnational policies, financing models, and regulatory environments that incentivize rapid outbreak response and sustain routine care.
- Health System Management: Optimizing human resources for health, supply-chain logistics, and facility-level coordination to maintain continuity of care during emergencies.
- Digital Health & Informatics: Deploying electronic surveillance platforms, mobile health (mHealth) tools, and data-analytics dashboards for real-time monitoring, early warning, and evidence-based decision-making.
- Surveillance & Laboratory Networks: Expanding laboratory capacity, sample-transport systems, and integrated One Health approaches to detect zoonoses and emerging pathogens.
- Community Engagement & Risk Communication: Leveraging regional partnerships, local governance, and culturally tailored messaging to build trust and promote preventive behaviors.
- Operational Research & Evaluation: Implementing outbreak simulations, performance metrics, and rapid-cycle evaluations to refine interventions and inform scalable best practices.
We welcome submissions that generate practical, scalable solutions for African health systems. By uniting insights from policymakers, health managers, informaticians, and frontline practitioners, this Collection aims to inform evidence-driven investments, strengthen preparedness capacities, and improve health outcomes across the continent.
Publishing Model: Open Access
Deadline: Jun 01, 2026
Advances in Large Language Models for Health Systems
This collection invites original research articles, reviews, and case studies at the intersection of Computational Intelligence (CI), Artificial Intelligence (AI), and healthcare informatics and healthcare records, with a focus on Large Language Models (LLMs) methods and architectures. The aim is to showcase innovative methodologies and applications that bridge cutting-edge CI techniques with real-world challenges in healthcare.
We welcome contributions that present novel algorithms, frameworks, and applications of AI and CI in areas such as, clinical decision support, healthcare informatics, and ethical or legal aspects of medical AI. Special attention will be given to interdisciplinary work that highlights translational potential, system-level integration, or demonstrates real-world deployment within health systems. This collection seeks to foster collaboration among researchers, clinicians, data scientists, and system designers to promote advances that will shape the future of intelligent health technologies.
Topics of Interest Include, but Are Not Limited To:
- Integration of LLMs with electronic health records (EHRs) and clinical decision support systems
- Ethical, legal, and regulatory considerations in deploying LLMs in healthcare
- Case studies demonstrating real-world implementation of LLMs in health systems
Any study solely on development of an LLM application in healthcare records maintenance or health informatics should have application analysis to support.
Keywords: Computational Intelligence, Artificial Intelligence, Large Language Models, Healthcare Informatics, Clinical Decision Support, Intelligent Medical Systems。
Publishing Model: Open Access
Deadline: Apr 04, 2026





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in