A hidden cost: The economic burden of zoonotic disease on livestock and food security
Published in Microbiology and Economics

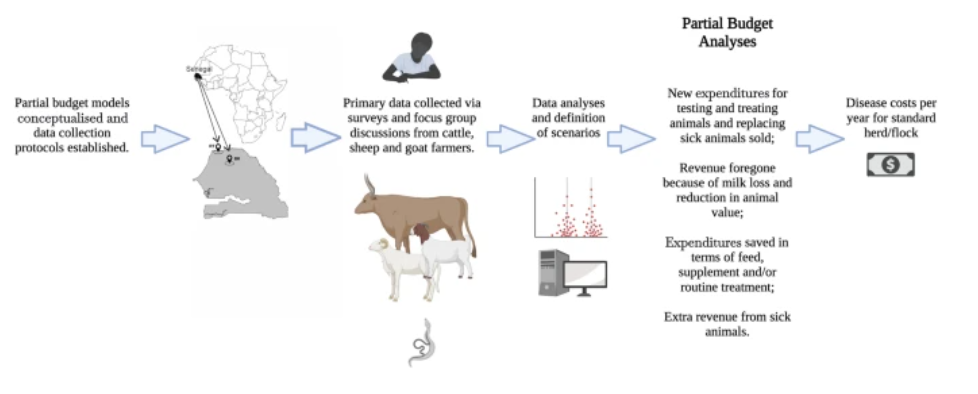
While writing a One Health blog on H5N1 avian influenza and Mpox, I read about the number of wild birds and farmed poultry dying from bird flu, coupled with the humane culling of whole flocks of poultry, and the rapid spread of the disease through cattle in the US. I also learnt about the £38 million that the vaccine alliance Gavi spent on vaccine doses for delivery to Mpox-infected countries in Africa after the disease was declared a public health emergency. These insights, along with local news stories like “Christmas turkeys killed early to avoid bird flu”, got me thinking about socioeconomic impacts of disease, an angle I often overlook. The financial burden of vector-borne and zoonotic diseases affects various groups, from farmers facing decreased productivity due to illness and death of animals, and individuals paying for healthcare or losing income due to working days lost; to healthcare systems and governments investing in monitoring, diagnosis, prevention and treatment of disease, investments that are important in the control of zoonotic diseases and pandemic prevention. Studies where the economic burden of a disease is measured across different sectors are important components of One Health.
Parasites and profits
Parasitism is a big threat to farms’ profitability and farmers’ sources of income. In cattle for example, parasites can lead to illness and death of individuals, reduced milk production, weight loss, and the transmission of diseases across the herd that may also threaten human health. With dairy consumption and production increasing, parasitism in dairy cattle can also threaten food security. With climate change and land use change altering parasite distribution, seasonality, and abundance, the risk of vector-borne diseases is increasing. A 2020 study reported the annual global costs of helminth infections in dairy cattle across Europe were €941 million, most of this cost is attributable to production loss, and a small amount to the cost of treatment. Parasite preventatives (prophylactics) can be used to help increase production and yields. Although there is a financial cost of obtaining these, the increase in productivity means that producers can make this money back and begin to see profits again.
A vector that is particularly costly to farmers is the tick. These ectoparasites negatively impact cattle production by causing blood loss, immune suppression, damaging and devaluing hides, and by transmitting pathogens. Agriculture is the source of income for many across Sub-Saharan Africa, and many farmers in this region are already subject to resource constraints which can affect their livelihoods, they need access to affordable parasite control to improve their yields. One study identifying tick species with high economic impact on cattle in this region noted that standardised surveys on tick exposure are necessary for governments and private institutions to create affordable diagnostics and invest in effective infection control. Around 70% of the cows this study sampled were infested with at least one tick species, and more commonly than not species were found co-infesting cattle. They observed spatial variation in tick prevalence and species distribution, meaning that control measures need to be tailored locally to optimise efficiency. Studies like this can help governments make successful, cost-effective decisions when investing in things like parasite control that can enable small-scale livestock farmers to remain profitable.

Heylen, D.J.A., Kumsa, B., Kimbita, E. et al. Tick communities of cattle in smallholder rural
Livestock production systems in sub-Saharan Africa. Parasites Vectors 16, 206 (2023).
Schistosomiasis in livestock
Schistosomiasis is a vector-borne disease that infects both mammals and humans, it is known to be one of the most devastating diseases in terms of economic impact yet few studies estimate the financial impact the disease has on farmers when it infects livestock. In Sub-Saharan Africa, large-scale mass drug administration with praziquantel in humans is used to control morbidity. The drug is donated by Merck who in collaboration with WHO supply 250 million tablets annually however, if there is no complementary control of the disease in livestock this hinders efforts to control the disease and overlooks the burden that animal infection can have on smallholders.
Research on diagnosis and treatment costs and the economic impact of the disease on farmers could help decision makers to conduct cost effectiveness analyses and implement effective disease control methods. For example, a study in the 80s estimated that in areas with high schistosomiasis infection and high mortality, having a high percentage of animals vaccinated with low-cost vaccines would give a high return on investments, i.e. effective low-cost treatment could substantially increase productivity and output. Currently, the lack of access to a veterinary formula and dose of PZQ means that some farmers use donated human PZQ to treat their animals. In these cases, doses are often incorrect so do not help to increase productivity, and most alarmingly, the misuse can lead to drug resistance. Pharmaceutical companies and governments therefore need to find cost-effective schistosomiasis vaccines for livestock.
Conclusion
The economic impacts of vector borne diseases in livestock are wide-ranging, affecting different groups and sectors at various levels. The biggest hit is undoubtedly felt by farmers who experience loss of livestock, substantial decreases in productivity, and whose livelihoods are put at risk. There are many benefits to encouraging different sectors to come together to research and invest in efficient cost-effective parasite and disease control in livestock including making farmers’ income more sustainable, helping to ensure food security and controlling the transmission of disease and curb zoonotic spillover.
Poster image from Wikimedia- Rasheedhrasheed. Licensed under the Creative Commons Attribution-Share Alike 4.0 International license.
Editing and title suggestion by Daniel Parsons!
Follow the Topic
-
Parasites & Vectors

This journal publishes articles on the biology of parasites, parasitic diseases, intermediate hosts, vectors and vector-borne pathogens.
-
BugBitten
A blog for the parasitology and vector biology community.
Related Collections
With Collections, you can get published faster and increase your visibility.
Credelio Quattro
Articles published in the collection have already gone through the systematic peer review process of the journal.
Publishing Model: Open Access
Deadline: Apr 24, 2026
Translational leishmaniasis
Topics of interest include:
• Analytical and omics-based advances for parasite detection, monitoring, and characterization.
• Comparative biology of Leishmania and related parasites, with emphasis on shared mechanisms of infection and survival.
• Host-pathogen interactions and immune responses.
• Epidemiology and transmission dynamics, including drawing parallels between leishmaniasis and other parasitic infections.
• Translational strategies in drug discovery, vaccine development, and therapeutic interventions applicable to leishmaniasis and related parasitic diseases.
By linking leishmaniasis with other parasitic diseases of similar characteristics, this collection seeks to foster integrative approaches, stimulate cross-disciplinary dialogue, and accelerate the translation of knowledge into innovative diagnostic, preventive, and therapeutic solutions.
Publishing Model: Open Access
Deadline: Jun 01, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in