A novel hybrid method with convergence analysis for approximation of HTLV-I dynamics model
Published in Mathematics and Statistics
A Novel Numerical Approach to Solving the HTLV-I T-Cell Infection Model
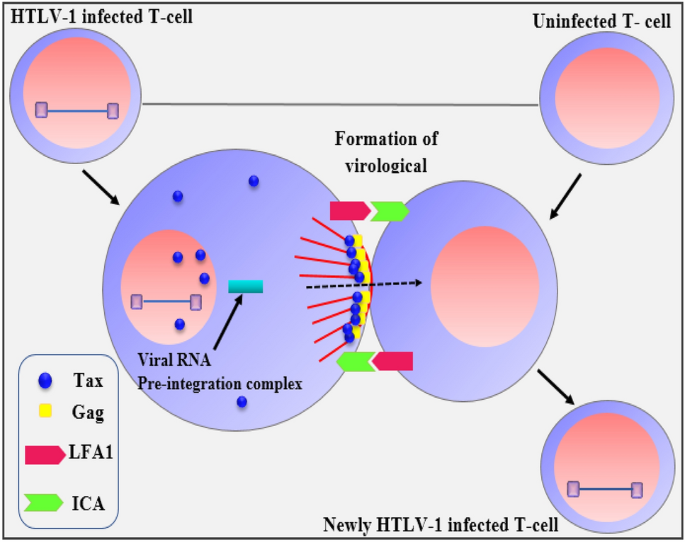
This paper presents a groundbreaking numerical approach aimed at approximating solutions to the mathematical model describing the infection of T-cells by the human T-cell lymphotropic virus I (HTLV-I). HTLV-I is a retrovirus that infects T-cells, a crucial component of the human immune system and is associated with a range of diseases, including adult T-cell leukemia/lymphoma and HTLV-I-associated myelopathy. The accurate modeling of this infection is essential for understanding the dynamics of viral propagation and the immune response. However, the fractional nature of the differential equations used to describe this system adds complexity to solving the model, making robust numerical methods imperative.
In this study, we propose a hybrid method that combines the operational matrix technique and the spectral method to transform the fractional model into a solvable system of nonlinear algebraic equations. Fractional differential equations are widely recognized for their ability to model processes exhibiting memory and hereditary properties, which are intrinsic to many biological systems. These equations, however, pose significant computational challenges, especially when their analytical solutions are unattainable. The proposed approach addresses these challenges effectively, offering a practical framework for approximating the solutions.
The operational matrix method plays a critical role in reducing computational complexity by transforming the fractional derivatives in the model into operational matrices. This transformation simplifies the problem, enabling its representation as a finite-dimensional system. The spectral method complements this approach by leveraging the orthogonality of basis functions to achieve high accuracy in approximating the solution. This synergy between the operational matrix and spectral methods forms the backbone of the proposed numerical approach.
One of the significant highlights of this paper is the use of the Levenberg-Marquardt algorithm to solve the resulting system of nonlinear algebraic equations. The Levenberg-Marquardt algorithm is a robust optimization technique widely used for solving problems involving nonlinear least squares. Its adaptability to the current framework ensures efficient convergence, even in the presence of the inherent complexities of the fractional model. This combination of numerical strategies enhances the reliability and efficiency of the solution process.
The theoretical contributions of this study are equally noteworthy. A detailed convergence analysis is conducted to establish the validity of the proposed method. Convergence analysis provides mathematical assurance that the numerical solution approximates the exact solution as the computational effort increases. Additionally, the paper derives error bounds, which offer quantitative measures of the solution's accuracy. These theoretical guarantees underpin the robustness of the proposed numerical approach and demonstrate its potential for broader applications in modeling biological systems.
To assess the effectiveness of the method, we apply it to several test problems derived from the HTLV-I T-cell infection model. These problems are carefully selected to reflect the diverse dynamical behaviors of the system. The numerical results showcase the proposed method's accuracy and efficiency, even in scenarios characterized by rapid changes in the state variables or high sensitivity to parameter variations. The performance of the method is benchmarked against existing approaches reported in the literature, providing a comparative perspective on its reliability and computational advantages.
The results of this comparative analysis are compelling. The proposed method consistently outperforms traditional techniques in terms of accuracy, computational efficiency, and robustness. These findings highlight its suitability for solving complex fractional differential equations, particularly those arising in the context of biological and medical modeling. The method's ability to handle the intricacies of the HTLV-I T-cell infection model underscores its potential as a valuable tool for researchers and practitioners in the field.
Moreover, this study's implications extend beyond the immediate context of HTLV-I modeling. Fractional differential equations are ubiquitous in science and engineering, with applications ranging from fluid dynamics and signal processing to epidemiology and population dynamics. The proposed approach offers a versatile framework that can be adapted to tackle a wide range of problems involving fractional systems. Its generalizability and scalability make it a promising candidate for addressing emerging challenges in various domains.
In conclusion, this paper introduces a novel numerical approach that bridges the gap between theoretical innovation and practical application in solving fractional differential equations. By integrating the operational matrix method with the spectral method and employing the Levenberg-Marquardt algorithm, we achieve a powerful computational framework for modeling the dynamics of HTLV-I T-cell infection. The theoretical convergence analysis and error bounds reinforce the method's validity, while the numerical experiments demonstrate its superior performance compared to existing techniques. These findings pave the way for future research to refine and expand the proposed approach's applicability to other complex systems.
The significance of this work lies not only in its contribution to the mathematical modeling of HTLV-I T-cell infection but also in its potential to inspire advancements in numerical methods for fractional differential equations. Researchers, mathematicians, and computational scientists are encouraged to build upon this foundation, exploring new avenues for innovation and application. The proposed method stands as a testament to the power of interdisciplinary collaboration, where mathematical ingenuity meets the pressing challenges of biomedical research.
Follow the Topic
-
Scientific Reports

An open access journal publishing original research from across all areas of the natural sciences, psychology, medicine and engineering.
Related Collections
With Collections, you can get published faster and increase your visibility.
Obesity
Publishing Model: Hybrid
Deadline: Apr 24, 2026
Reproductive Health
Publishing Model: Hybrid
Deadline: Mar 30, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in