Exploring donor difference in neonatal fecal microbiota transplantation
Published in Microbiology
Fecal microbiota transplantation (FMT) shows high cure rates (90% on average) in treating Clostridioides difficile infection1, the success of which is even not decided by a specific donor2. This overwhelming success encouraged researchers to expand the list of FMT use. Unfortunately, donor difference appears when FMT is applied to multi-factorial diseases, e.g., inflammatory bowel disease, and metabolic syndrome.
Necrotizing enterocolitis (NEC) is a devasting neonatal bowel disease implicated by immune imbalance, exogenous intake (e.g., formula feeding), gut dysbiosis, and other factors. First reported in the early 1800s3, NEC has an incidence of up to 7% among infants born preterm4. For very-low-birth-weight infants, the mortality rate can go up to 10-30%, which has not improved for years despite the aid of antibiotics or probiotics4.
We have found that FMT could reduce NEC incidence in preterm piglets while inhibiting mucosal pro-inflammatory TLR4 signaling5. However, in a follow-up study, we could not replicate the finding with a donor collected from another farm. This gave us a chance to explore the donor difference in FMT efficiency in a controlled environment.
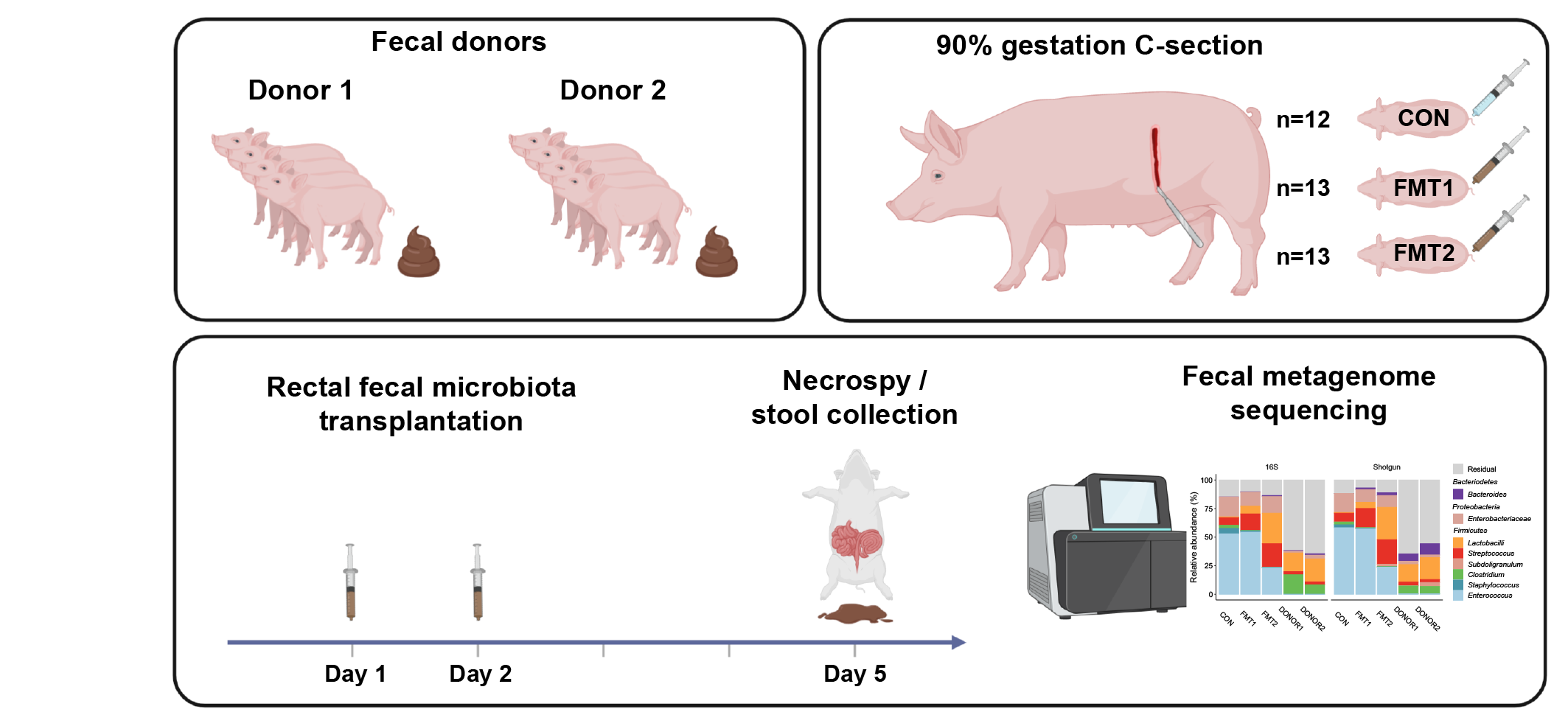
As shown in Fig. 1, the two donors were collected from two different specific pathogen-free herds. Preterm piglets at 90% gestation age, as recipients, received rectal FMT of the two donors or sterile saline in their first two days of life. Although the two donor communities showed comparably similar bacterial diversity and composition at the genus level, they differed in their efficiency in protecting the gastrointestinal tract of recipient piglets. Relative to the other, Donor 2 showed a superior effect in preventing NEC incidence and ameliorating diarrhea symptoms among preterm piglets (Fig. 2a). Meanwhile, the metagenomic analysis revealed that the administered donor is the driving factor in shaping the gut microbiome structure of recipients (Fig. 2b). Relative to sham-treated animals, a significant increase in lactobacilli and reductions in Enterobacteriaceae were found in the gut of FMT recipients, especially among those receiving the effective donor.
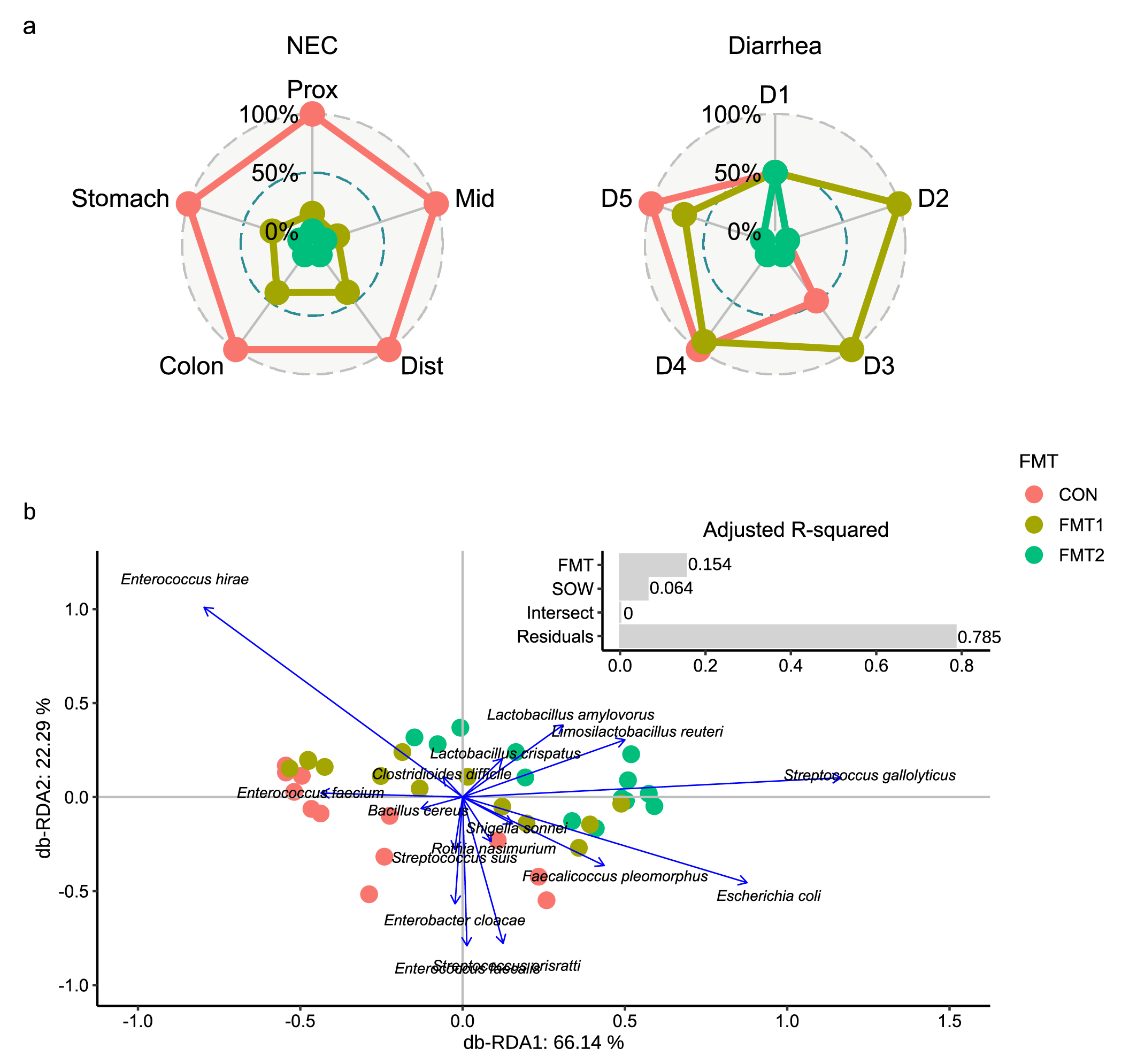
Interestingly, the lactobacilli engraftment showed a distinct donor-dependent tendency. The lactobacilli population from Donor 2 seemed to have more efficient colonization (Fig. 3a) and propagation (Fig. 3b) in the gut after FMT. Phylogenetic tracing indicated a clear strain-level separation between the engrafted lactobacilli, which consistently corresponded to the given donors. A logical inference is that the variation of stains in donors affected the bacterial colonization in recipients, and in turn the clinical response.
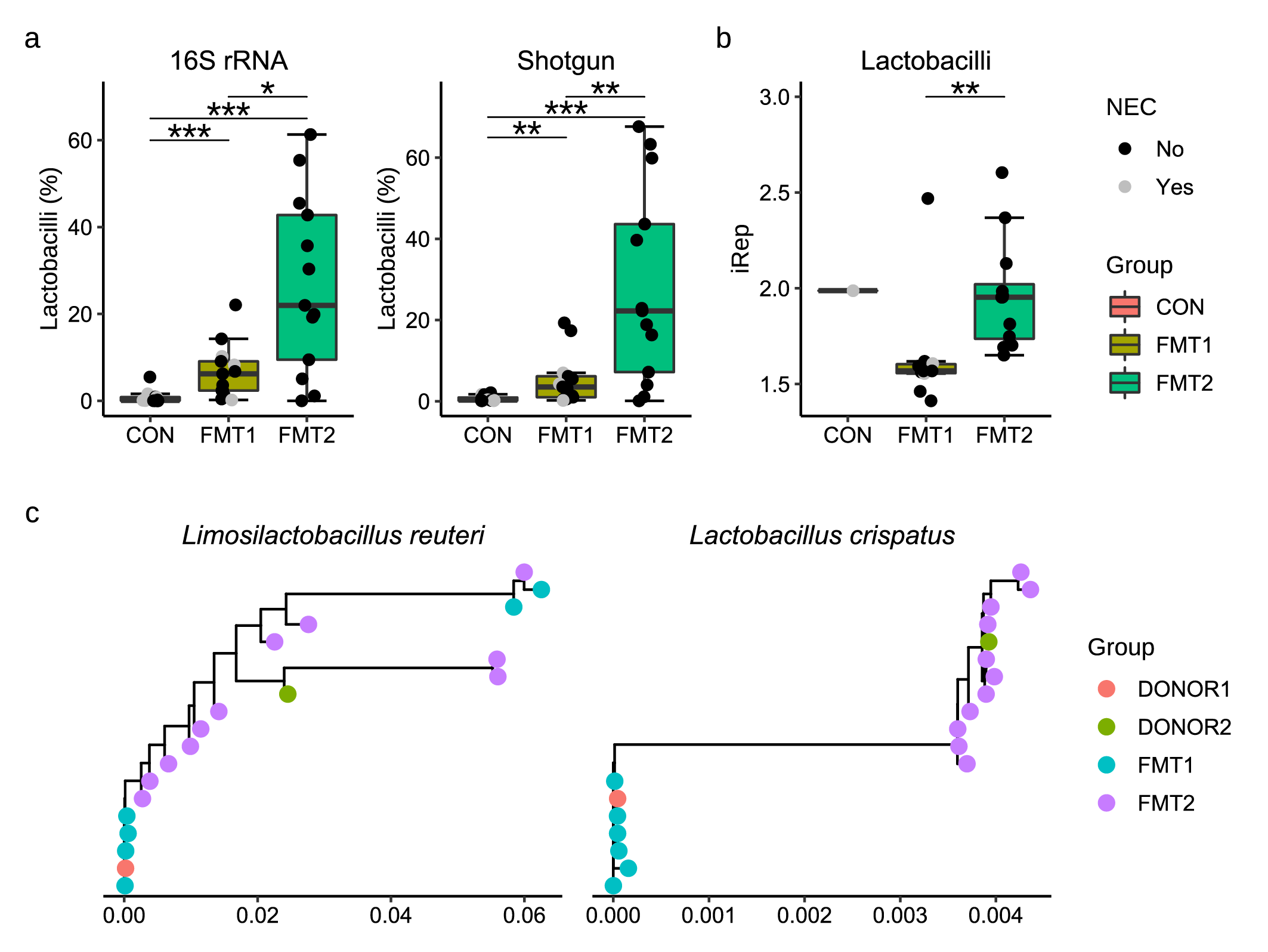
Fig. 3. Donor-dependent lactobacilli engraftment and phylogenetic separation in the recipient piglets. The colonization (a), replication rate (b), and phylogenetic similarity of lactobacilli in recipients were decided by the received donors.
Due to the risk of antibiotic resistance and uncertainty regarding the effects of probiotic treatment, FMT could be a promising direction for microbiome-targeted therapy. Donor choice is considered essentially necessary in FMT as accumulated evidence disclosed the super donor phenomenon in treating polygenic diseases including NEC. Donor species richness is reported to be a crucial factor for the success of FMT treatment in inflammatory bowel disease6. In this study, the discrepant efficiency from two comparable donors, at least suggested donor diversity could not alone ensure a favorable outcome of FMT. Looking into the donor composition, we found the strain-level variation between donors might affect the bacterial engraftment and therefore the treatment results. The lactobacilli strains from the two donors showed phylogenetic differences and differed in inhabiting the gut of recipients. The effective donor led to efficient engraftment of lactobacilli in rapid replication rate, greatly inhibiting pathogens e.g., Enterobacteriaceae overgrowth, and reducing the potential gastrointestinal disorders. Moreover, a glycosaminoglycans-degrading Bacteroides genome was recovered from the effective donor, which possibly contributed to pathogen resistance through competing for binding sites along the host gut epithelial extracellular matrix. Besides, we noticed increased pathogenetic signatures of Escherichia coli and Salmonella enterica in the gut of recipients receiving either of the donors. The transferred pathogens didn’t violate the FMT outcome of the efficient donor but initiated high sepsis cases in another oral FMT study5. This again highlights the importance of donor selection in FMT therapy.
By
Yan Hui, Anders Brunse, Dennis Sandris Nielsen
Read the full paper at: https://www.nature.com/articles/s41522-022-00310-2
Bibliography
- Osman, M. et al. Donor Efficacy in Fecal Microbiota Transplantation for Recurrent Clostridium difficile: Evidence From a 1,999-Patient Cohort. Open Forum Infectious Diseases 3, (2016).
- Kassam, Z., Lee, C. H., Yuan, Y. & Hunt, R. H. Fecal Microbiota Transplantation for Clostridium difficile Infection: Systematic Review and Meta-Analysis. American Journal of Gastroenterology 108, 500–508 (2013).
- Obladen, M. Necrotizing Enterocolitis - 150 Years of Fruitless Search for the Cause. Neonatology 96, 203–210 (2009).
- Neu, J. & Walker, W. A. Necrotizing Enterocolitis. New England Journal of Medicine 364, 255–264 (2011).
Follow the Topic
-
npj Biofilms and Microbiomes

The aim of this journal is to serve as a comprehensive platform to promote biofilms and microbiomes research across a wide spectrum of scientific disciplines.
Related Collections
With Collections, you can get published faster and increase your visibility.
Natural bioactives, Gut microbiome, and human metabolism
Publishing Model: Open Access
Deadline: May 20, 2026
Harnessing plant microbiomes to improve performance and mechanistic understanding
Publishing Model: Open Access
Deadline: Jun 01, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in