Tepotinib in Asian patients with METex14 skipping NSCLC
Published in Cancer and General & Internal Medicine
METex14 skipping NSCLC and tepotinib
Mutations in the mesenchymal–epithelial transition (MET) gene drive the growth of some subtypes of non-small cell lung cancer (NSCLC). MET encodes a tyrosine kinase receptor for hepatocyte growth factor. Internalization and degradation of the receptor involves binding of a key intracellular region encoded by exon 14. METex14 skipping splice site mutations cause loss of this region, resulting in accumulation of MET and oncogenic downstream signaling.1
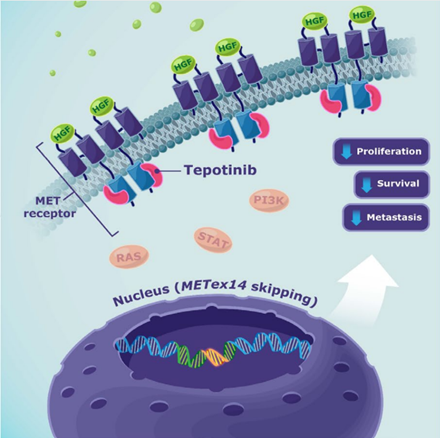
METex14 skipping mutations cause excess of MET receptor at the cell surface. Tepotinib inhibits tyrosine kinase activity of the receptor, reducing signaling in the hepatocyte growth factor pathway. Image © Merck, reproduced with permission.
METex14 skipping occurs in 3–4% of patients with NSCLC, including 1–4% of Asian patients with lung adenocarcinoma.1–7 Several selective MET inhibitors have demonstrated clinical activity in patients with METex14 skipping NSCLC,2 including tepotinib.8,9 This oral, once-daily, highly selective, potent MET inhibitor is currently approved for treating advanced or metastatic METex14 skipping NSCLC in many countries in Europe, North America, South America, and Asia, and is the first MET inhibitor with full approval in China. The US FDA granted full approval, in place of the previous accelerated approval, in February 2024.10
Detection of METex14 skipping
METex14 skipping can be detected via the complementary approaches of tumor tissue biopsy (TBx) or liquid biopsy (LBx).11 TBx is the more established technique and involves detection of DNA or RNA in tumor biopsy samples. LBx offers several advantages over TBx, including being more convenient and less invasive. LBx measures levels of circulating tumor DNA in plasma and its use is increasing, particularly when TBx samples are inadequate or unavailable.11–13 The dynamics of consecutive LBx measurement may also be used to monitor response and progression to treatment in NSCLC.9,11,14,15
VISION trial of tepotinib
The basis for regulatory approval of tepotinib was the Phase II VISION study (NCT02864992), which included two cohorts, A and C, of patients with METex14 skipping advanced/metastatic NSCLC as detected by TBx and/or LBx.9,16 In these combined groups (totaling 313 patients, 62% white and 34% Asian), robust and durable clinical activity of tepotinib was demonstrated in long-term follow-up (median 32.6 months [range: 0.3–71.9]; data cut-off: November 20, 2022), with an objective response rate (ORR) of 51.4% (95% confidence interval [CI]: 45.8, 57.1), median duration of response (DOR) of 18.0 months (95% CI: 12.4, 46.4), and median progression-free survival (PFS) of 11.2 months (95% CI: 9.5, 13.8).17
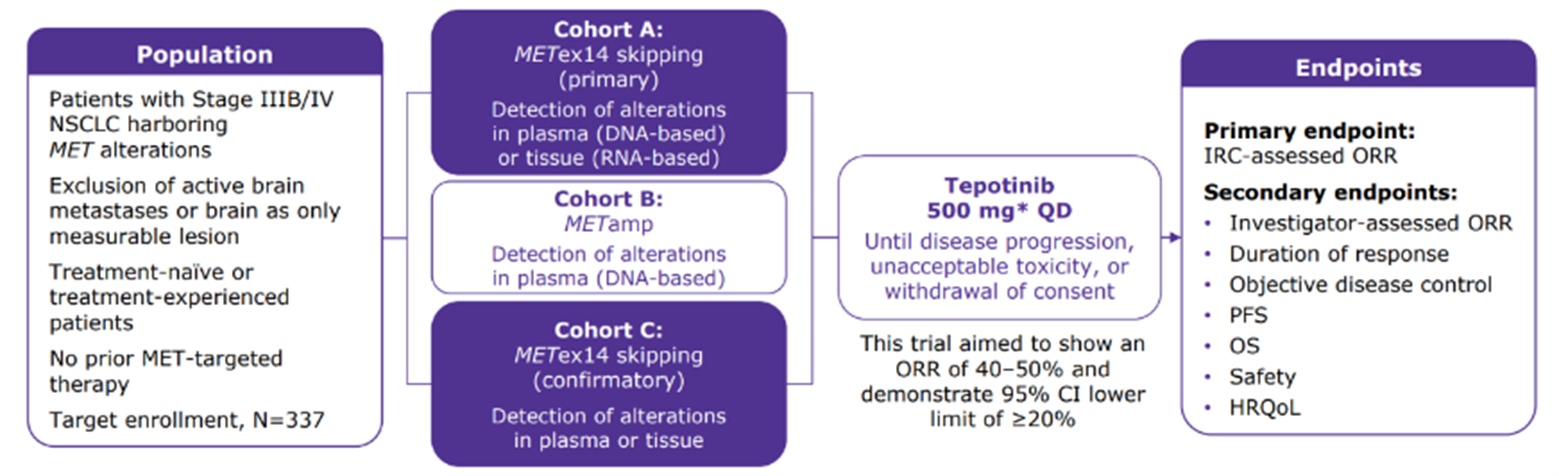
The VISION study included two cohorts, primary cohort A and confirmatory cohort C, of patients with METex14 skipping NSCLC, identified by either LBx or TBx
Asian patients in VISION
Genetic or environmental factors, including regional differences in clinical practice, have been proposed to influence the performance of anticancer drugs between patients from different ethnic groups.18 For NSCLC, differences in epidemiology, clinicopathologic characteristics, and prognosis have been reported between Asian and non-Asian populations.19
Our analysis included the 106 Asian patients from the METex14 cohorts in VISION who were treated with tepotinib and had at least 18 months of follow-up. The group constitutes the largest population of Asian patients with METex14 skipping NSCLC in a MET inhibitor trial to date, comprising 38 patients from Japan, 20 from South Korea, 12 from Taiwan, 30 from China, and six enrolled from outside Asia. Almost half (47.2%) had received no previous treatment while the rest had up to two previous treatments (patients previously treated with MET inhibitors were not eligible).
Outcomes in Asian patients in VISION
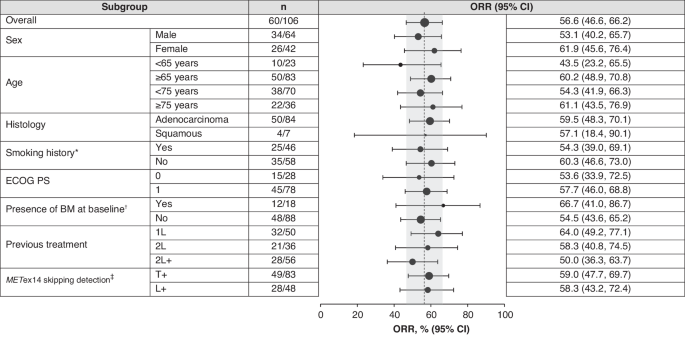
Efficacy of tepotinib was similar for Asian patients as for the global study population, with an ORR of 56.6% (95% CI: 46.6, 66.2). As might be expected, ORR was higher in patients with no prior treatment (64.0% [95% CI: 49.2, 77.1]) than in previously treated patients (ORR 50.0% [95% CI: 36.3, 63.7]). Nonetheless, these robust efficacy data from Asian patients in VISION support the use of tepotinib in first or subsequent lines of therapy in patients with METex14 skipping metastatic NSCLC, which is in line with recently published consensus from the Asian Thoracic Oncology Research Group (ATORG).12
The most common treatment-related adverse events (TRAEs) in the Asian population in the VISION study were peripheral edema (a MET inhibitor class effect), creatinine increase, and diarrhea. Tepotinib was generally well tolerated in Asian patients, with a low proportion of TRAEs leading to discontinuation. Health-related quality of life (HRQoL) remained stable during treatment, the first time HRQoL has been examined in Asian patients with METex14 skipping NSCLC treated with a MET inhibitor.
Other MET inhibitors besides tepotinib include capmatinib and savolitinib (in China), which are included in the ATORG consensus recommendations,12 and gumarontinib, which has also demonstrated efficacy in Asian patients with METex14 skipping NSCLC.20–22 As we discuss in our paper, available data support the use of MET inhibitors in this population, and our findings compare favorably with those of these other agents.
Use of LBx in VISION
In VISION, 83 Asian patients were enrolled by TBx, and 48 by LBx. Results were similar for those identified via either method, with ORR of 59.0% (95% CI: 47.7, 69.7) in the TBx group and 58.3% (95% CI: 43.2, 72.4) for LBx. Despite this consistency, there were trends in the LBx group to shorter median PFS in previously treated patients and shorter median OS irrespective of prior treatment. These findings were concordant with the overall study, and may reflect a poorer prognosis of the LBx subgroup due to a greater baseline disease burden.23 Tumor size correlates with shedding of circulating tumor DNA,24 so LBx may preferentially identify patients with higher tumor load. Overall, TBx and LBx both seem to be appropriate methods for identifying patients likely to benefit with tepotinib.
Summary
Our analysis confirms effiacy and safety of tepotinib in Asian patients with METex14 skipping NSCLC, with stability of HRQoL. Whether patients are identified via LBx or TBx, these data support use of tepotinib in first or subsequent lines of therapy to improve clinical outcomes.
1 Awad MM, Oxnard GR, Jackman DM, Savukoski DO, Hall D, Shivdasani P et al. MET exon 14 mutations in non-small-cell lung cancer are associated with advanced age and stage-dependent MET genomic amplification and c-Met overexpression. Journal of Clinical Oncology 2016; 34: 721–730.
2 Salgia R, Sattler M, Scheele J, Stroh C, Felip E. The promise of selective MET inhibitors in non-small cell lung cancer with MET exon 14 skipping. Cancer Treat Rev 2020; 87: 102022.
3 Reungwetwattana T, Liang Y, Zhu V, Ou S-HI. The race to target MET exon 14 skipping alterations in non-small cell lung cancer: The Why, the How, the Who, the Unknown, and the Inevitable. Lung Cancer 2017; 103: 27–37.
4 Gow C-H, Hsieh M-S, Wu S-G, Shih J-Y. A comprehensive analysis of clinical outcomes in lung cancer patients harboring a MET exon 14 skipping mutation compared to other driver mutations in an East Asian population. Lung Cancer 2017; 103: 82–89.
5 Liu SY, Gou LY, Li AN, Lou NN, Gao HF, Su J et al. The unique characteristics of MET exon 14 mutation in Chinese patients with NSCLC. Journal of Thoracic Oncology 2016; 11: 1503–1510.
6 Xu Z, Li H, Dong Y, Cheng P, Luo F, Fu S et al. Incidence and PD-L1 Expression of MET 14 Skipping in Chinese Population: A Non-Selective NSCLC Cohort Study Using RNA-Based Sequencing. Onco Targets Ther 2020; 13: 6245–6253.
7 Lee GD, Lee SE, Oh DY, Yu DB, Jeong HM, Hong S et al. MET exon 14 skipping mutations in lung adenocarcinoma: Clinicopathologic implications and prognostic values. Journal of Thoracic Oncology 2017; 12: 1233–1246.
8 Falchook GS, Kurzrock R, Amin HM, Xiong W, Fu S, Piha-Paul SA et al. First-in-man Phase I trial of the selective MET inhibitor tepotinib in patients with advanced solid tumors. Clinical Cancer Research 2020; 26: 1237–1246.
9 Paik P, Felip E, Veillon R, Sakai H, Cortot AB, Garassino MC et al. Tepotinib in non-small-cell lung cancer with MET exon 14 skipping mutations. New England Journal of Medicine 2020; 383: 931–943.
10 Food and Drug Administration. FDA approves tepotinib for metastatic non-small cell lung cancer. 2024.https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-tepotinib-metastatic-non-small-cell-lung-cancer (accessed 23 Feb2024).
11 Rolfo C, Mack P, Scagliotti G V., Aggarwal C, Arcila ME, Barlesi F et al. Liquid Biopsy for Advanced NSCLC: A Consensus Statement From the International Association for the Study of Lung Cancer. Journal of Thoracic Oncology 2021; 16: 1647–1662.
12 Ahn MJ, Mendoza MJL, Pavlakis N, Kato T, Soo RA, Kim DW et al. Asian Thoracic Oncology Research Group (ATORG) Expert Consensus Statement on MET Alterations in NSCLC: Diagnostic and Therapeutic Considerations. Clin Lung Cancer 2022; 23: 670–685.
13 Esagian SM, Grigoriadou G, Nikas IP, Boikou V, Sadow PM, Won JK et al. Comparison of liquid-based to tissue-based biopsy analysis by targeted next generation sequencing in advanced non-small cell lung cancer: a comprehensive systematic review. J Cancer Res Clin Oncol 2020; 146: 2051–2066.
14 Mack PC, Miao J, Redman MW, Moon J, Goldberg SB, Herbst RS et al. Circulating Tumor DNA Kinetics Predict Progression-Free and Overall Survival in EGFR TKI–Treated Patients with EGFR-Mutant NSCLC (SWOG S1403). Clinical Cancer Research 2022; 28: 3752–3760.
15 Anagnostou V, Forde PM, White JR, Niknafs N, Hruban C, Naidoo J et al. Dynamics of tumor and immune responses during immune checkpoint blockade in non–small cell lung cancer. Cancer Res 2019; 79: 1214–1225.
16 Le X, Sakai H, Felip E, Veillon R, Garassino MC, Raskin J et al. Tepotinib efficacy and safety in patients with MET exon 14 skipping NSCLC: Outcomes in patient subgroups from the VISION study with relevance for clinical practice. Clinical Cancer Research 2022; 28: 1117–1126.
17 Mazieres J, Paik PK, Garassino MC, Le X, Sakai H, Veillon R et al. Tepotinib Treatment in Patients With MET Exon 14–Skipping Non–Small Cell Lung Cancer: Long-term Follow-up of the VISION Phase 2 Nonrandomized Clinical Trial. JAMA Oncol 2023; 9: 1260–1266.
18 O’Donnell PH, Dolan ME. Cancer pharmacoethnicity: ethnic differences in susceptibility to the effects of chemotherapy. Clin Cancer Res 2009; 15: 4806–4814.
19 Zhou W, Christiani DC. East meets West: ethnic differences in epidemiology and clinical behaviors of lung cancer between East Asians and Caucasians. Chin J Cancer 2011; 30: 287.
20 Han J, Wolf J, Garon E, Groen H, Heist R, Ang M et al. Capmatinib in Patients with METex14-Mutated Non-Small Cell Lung Cancer: GEOMETRY Mono-1 Asian Subgroup Analysis. Journal of Thoracic Oncology 2021; 16: S670.
21 Lu S, Fang J, Li X, Cao L, Zhou J, Guo Q et al. Long-Term Efficacy, Safety and Subgroup Analysis of Savolitinib in Chinese Patients with Non-Small Cell Lung Cancers Harboring MET Exon 14 Skipping Alterations. JTO Clin Res Rep 2022; 3: 100407.
22 Yu Y, Zhou J, Li X, Goto K, Min X, Nishino K et al. Gumarontinib in patients with non-small-cell lung cancer harbouring MET exon 14 skipping mutations: a multicentre, single-arm, open-label, phase 1b/2 trial. EClinicalMedicine 2023; 59. doi:10.1016/j.eclinm.2023.101952.
23 Rolfo CD, OBrate Grupp AM, Menzel C, Bruns R, Juraeva D, Stroh C et al. 1382P Liquid biopsies (LBx) and tissue biopsies (TBx) for identifying MET exon 14 (METex14) skipping in advanced NSCLC: Analyses from the phase II VISION study of tepotinib. Annals of Oncology 2023; 34: S793.
24 Lam VK, Zhang J, Wu CC, Tran HT, Li L, Diao L et al. Genotype-Specific Differences in Circulating Tumor DNA Levels in Advanced NSCLC. Journal of Thoracic Oncology 2021; 16: 601–609.
Follow the Topic
-
British Journal of Cancer

This journal is devoted to publishing cutting edge discovery, translational and clinical cancer research across the broad spectrum of oncology.
Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in