“Thermopylae path” of the Brain-gut-microbiota axis: acute central stress damage the colonocytes’ mitochondria to disrupt the gut microbiota
Published in Microbiology
Explore the Research
Stress triggers gut dysbiosis via CRH-CRHR1-mitochondria pathway
npj Biofilms and Microbiomes - Stress triggers gut dysbiosis via CRH-CRHR1-mitochondria pathway
Battle of Thermopylae (480 BCE), which is a famous war in central Greece at the mountain pass of Thermopylae. The great Spartan King Leonidas and his only 300 warriors successfully resisted the invasion of Persian king Xerxes I and his vast army. However, Ephialtes, a Greek citizen desiring reward, informed Xerxes of a path that went around Thermopylae, thus smashing the Spartans’ line. Just as this famous war, there also a secret way exists under the expanse and complex Brain-gut-microbiota axis, which is the CRH-CRHR1-mitochondria pathway.
Psychological stress has been implicated in brain-gut communication disorder and gut dysbiosis, both of which are closely associated with the development and progression of gastrointestinal diseases, notably IBS. However, crucial questions persist regarding how remote brain stressors are sensed at the gut interface and the specific signaling pathway that mediate control over gut microbiota. The recent study has uncovered a novel mechanism involving colonocytes’ mitochondria in relaying stress signals to induce gut dysbiosis.
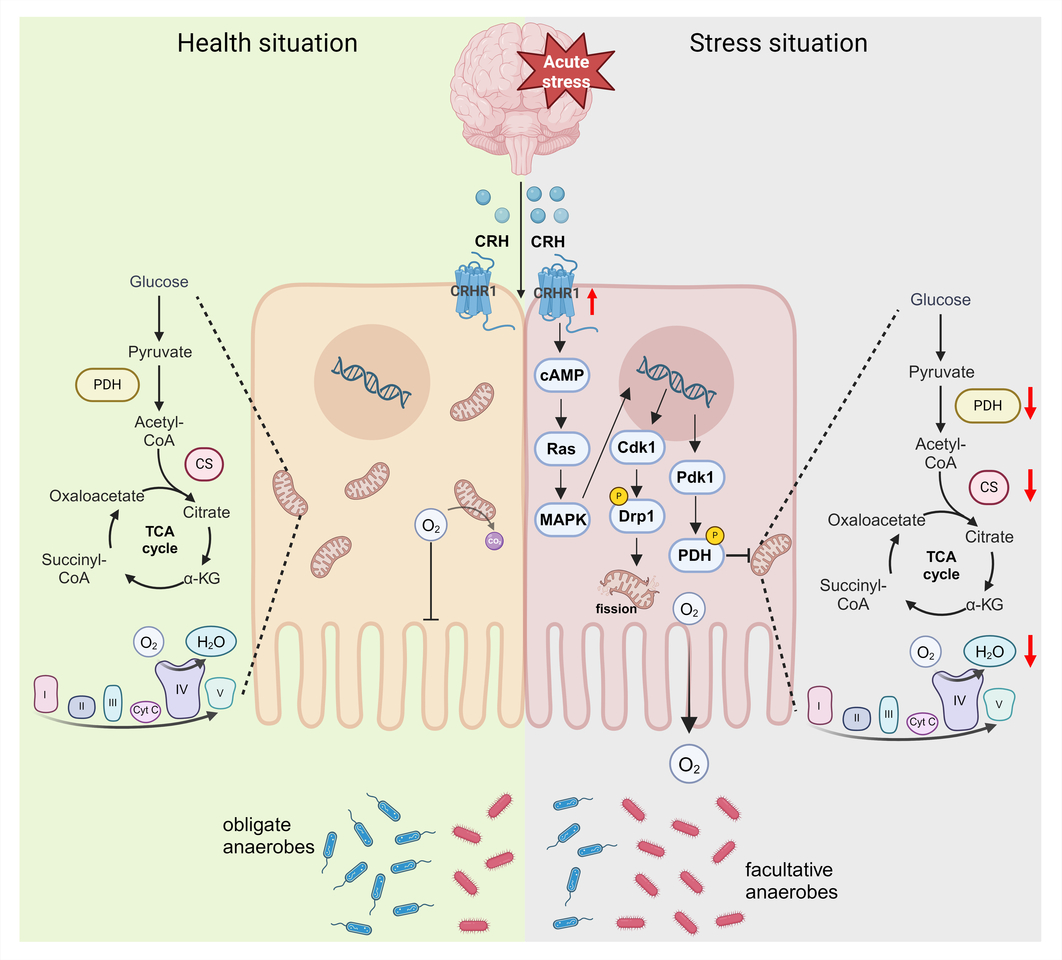
In this study, patients with IBS exhibited a significantly increase in facultative anaerobes and a decrease in obligate anaerobes, correlating with elevated serum corticotropin-releasing hormone (CRH) level and defective colonocytes’ mitochondria ultrastructure. Similarly, mice exposed to acute stress displayed enhanced CRH-CRH receptor type 1 (CRHR1) signaling, leading to impaired mitochondria function and epithelium hypoxia in the colon. Notably, colonocytes’ mitochondria is important for maintaining the colonic surface in a state of physiological hypoxia, which limits the amount of oxygen emanating from the mucosal surface, thereby checking aerobic growth of facultative anaerobes. Conversely, reduced mitochondria bioenergetics lower epithelial oxygen consumption, thereby diffusion of oxygen into the intestinal lumen. Based on these findings in both IBS patients and acutely stressed mice, we hypothesized that stress is transmitted from the brain to the gut via the CRH-CRHR1-mitochondria pathway, resulting in gut dysbiosis.
To test this hypothesis, we employed two distinct methodologies. By antagonizing CRHR1 expression or activating mitochondria respiration, we observed resilience against CRH-induced mitochondria damaging and epithelial hypoxia impairment, ultimately improving gut dysbiosis. Specifically, mice treated with CRH exhibited a dramatical increase in the abundance of facultative anaerobes, such as Pseudomonadaceae and Enterobacter, and a decrease in obligate anaerobes, such as Bacteroidaceae and Propionibacteriaceae, compared to pre-intervention mice. Notably, the growth of Pseudomonadaceae or Enterobacter can induce intestinal inflammation and intestinal barrier dysfunction through their virulence factor, such as exotoxins and hemolysins, while the decline of Bacteroidaceae and Propionibacteriaceae restricts the production of beneficial metabolites, such as acetic acid and propionic acid. However, mice treated with CRH combined with NBI (CRHR1 antagonist) or -5ASA (mitochondria agonist) inhibited the growth of facultative anaerobes while promoting the abundance of obligated anaerobes. It is worth noting that although either antagonizing CRHR1 or activating mitochondria can both improve CRH-induced mitochondria respiration reduction and epithelium hypoxia diminish, only antagonizing CRHR1 can restore mitochondria dynamics defects. For these reasons, antagonizing CRHR1 expression may be a more effective strategy than activating mitochondria to alleviate oxidative stress triggered by escaped mt-DNA or mt-ROS from damaged mitochondria. These results strongly suggest that the CRH-CRHR1-mitochondria pathway serves as a crucial mechanism for conveying psychological stress from the brain to the gut, thereby triggering gut dysbiosis.
Furthermore, we explored the mechanism underlying elevated CRHR1 expression-induced mitochondrial damage in colonocytes. To gain deeper insights into the underlying mechanisms associated with CRHR1 regulation and its impact on mitochondria, we conducted a transcriptome analysis on primary colon epithelial cells derived from mice. Our findings revealed upregulation of genes such as Camp, Mapk1, Cdk1 and Pdk1 in the CRHR1-activating group, while these genes were downregulated in the CRHR1-antagonizing group. Gene Set Enrichment Analysis (GSEA) further demonstrated significant regulation of pathways enriched in both the GO and KEGG databases, including upregulation of the "Ras signaling pathway" in the CRHR1-activating and its downregulation in the CRHR1-antagonizing group. Ultimately, our study demonstrates that CRHR1, in combination with CRH, activates the cAMP-Ras-MAPK signaling pathway. Subsequently, MAPK phosphorylate Drp1 via Cdk1, promoting mitochondrial fission and activating PDH kinase (Pdk1), which inhibits PDH activity and reduces mitochondrial respiration.
Our journey began with a long-standing question: how does stress trigger gut dysbiosis? We discovered that patients with IBS exhibit a distinct trend of gut microbiota changes, characterized by increased facultative anaerobes and decreased obligate anaerobes. To unravel this mystery, we employed metagenomics to analyze the microbiota, Oxygeaph-2k oroboros (O2K) technology and Seahorse Xfe24 technology to detect mitochondria respiration, and non-invasive micro-test technology (NMT) to reflect colon oxygen absorption in vivo. Surprisingly, we found that colonocytes’ mitochondria, a tiny cellular organelle, play a pivotal role in conveying stress from the brain to the gut and reshaping the gut microbiota composition.
Our laboratory has long been dedicated to researching gastrointestinal diseases and gut microbiota. We sincerely hope that our study will provide promising targets for the development of novel therapeutic strategies to manage stress-indued gastrointestinal diseases.
For this interested in the complete details of our research, please refer to our full paper, “Stress triggers gut dysbiosis via CRH-CRHR1-mitochondria pathway”, published in npj Biofilms and Microbiomes.
Follow the Topic
-
npj Biofilms and Microbiomes

The aim of this journal is to serve as a comprehensive platform to promote biofilms and microbiomes research across a wide spectrum of scientific disciplines.
Related Collections
With Collections, you can get published faster and increase your visibility.
Natural bioactives, Gut microbiome, and human metabolism
Publishing Model: Open Access
Deadline: May 20, 2026
Harnessing plant microbiomes to improve performance and mechanistic understanding
Publishing Model: Open Access
Deadline: Jun 01, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in