Topoisomerase I poison-triggered immune gene activation is markedly reduced in human small-cell lung cancers by impairment of the cGAS/STING pathway
Published in Cancer
Among all forms of tumors, human lung cancer has a high incidence and is the leading cause of cancer deaths. Lung cancer is constituted by different diseases distinguishable based on genetics, molecular and biological features. Small cell lung cancer (SCLC) is initially sensitive to chemotherapy and radiotherapy, which are current standard treatment, but recurrent tumors are often unresponsive to further treatments. Approved initial therapy includes cisplatin and etoposide or irinotecan, while only topotecan has been approved for relapsed SCLC.
The natural alkaloid camptothecin (CPT), its derivatives (topotecan and irinotecan) and synthetic indenoisoquinolines, are effective antitumor drugs which target DNA topoisomerase I (TOP1) [1–4]. They selectively target TOP1 in living cells by forming transient DNA-TOP1 cleavage complexes (TOP1ccs) leading to an increase of transcription/replication conflicts, R-loop formation, DNA damage accumulation, cell apoptosis and genome instability [1,5,6]. Interestingly, poisoning of TOP1 by CPT can increase mitotic errors and micronuclei [7–9]. Micronuclei are portions of chromatin or chromosomal fragments that are excluded from main nuclei and enclosed by nuclear membranes. Micronuclei frequency is generally increased in cancer cells as compared to normal cells, and is considered a marker of genome instability. Micronuclei were shown to mediate the activation of type I Interferon (IFN) and IFN-stimulated genes (ISG) in cancer cells through the cGAS-STING signaling pathway [10,11]. However, the underlying mechanism of micronuclei formation triggered by TOP1 poisons is not fully established.
Here, we have uncovered aspects of micronuclei formation by TOP1 poisons in cancer cells and the molecular defects of human SCLC that largely impair innate immune gene activation by TOP1 poisons. The findings highlight genetic and molecular features of human SCLC that can be exploited in patient stratification improving precision medicine strategies.
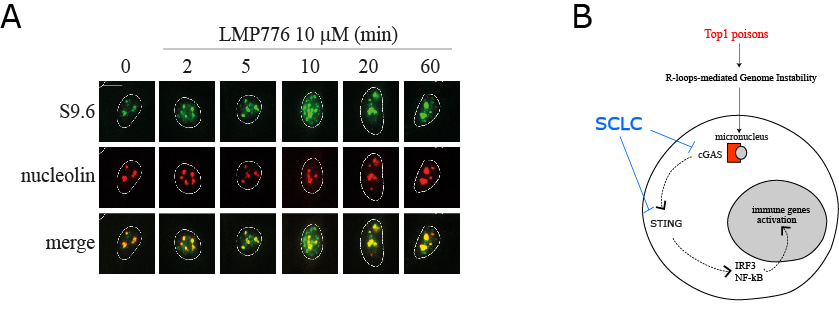
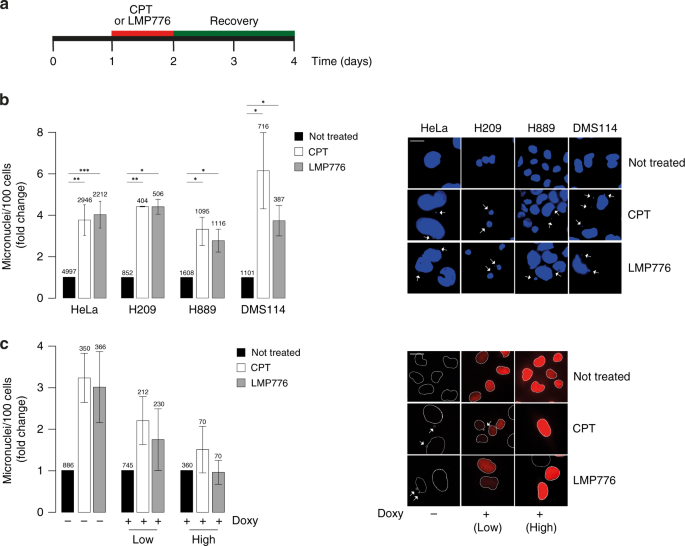
We initially have determined the levels of micronuclei in human SCLC (H209, H889 and DMS114) and HeLa cells treated with low doses of Top1 poisons (CPT and LMP776). Micronuclei were present in untreated cells at very similar levels in all the studied cell lines and the findings indicate that micronuclei formation by TOP1 poisons is likely a consistent phenomenon in many cancer cells. Next we determined whether R-loops could have a role in mediating micronuclei formation. Micronuclei induced by both CPT and LMP776 were reduced in RNase H1 overexpressing cells in comparison with wt cells. In addition, TOP1 poisons could trigger an increase of R-loops in cells as there is an increased hybrid signal in the nucleoplasm but not in nucleoli of cells treated for a few minutes to 1 hour with the TOP1 poisons, stained by IF with S9.6 (Ab against DNA:RNA hybrids) and an antibody against nucleolin (Figure 1A). As genome instability can derive from DNA damage, we then wondered whether the observed increase of R-loops can mediate DNA damage induced by CPT and LMP776. We found that RNaseH1 expression reduced and almost abolished CPT and LMP776-induced γH2AX foci demonstrating that TOP1 poison-induced DNA damage is mediated by R-loops.
Next, we determined the recruitment of cGAS to micronuclei with IF microscopy and the time course of cGAMP levels in HeLa cells exposed to CPT or LMP776 with an ELISA assay. These results show that CPT- and LMP776-triggered micronuclei can recruit and activate cGAS leading to an increase of the signaling molecule cGAMP. In addition, we used IF microscopy to detect STING in treated vs. untreated cells. The results showed that STING is localized in the perinuclear region of CPT- and LMP776-treated HeLa cells. The IF signal is therefore consistent with the mobilization of activated STING to the Golgi apparatus. As STING leads to the activation of inducible transcription factors, we determined the expression levels of immune genes (mainly IRF3 and NF-kB dependent) in HeLa cells treated with CPT or LMP776 and found that TOP1 poisons induced the expression of almost all the studied genes. Following STING silencing there is a strong reduction in transcription activation of both NF-kB- and IRF3-inducible genes demonstrating that TOP1 poison-induced immune gene activation is mainly due to the STING signaling pathway. Taken altogether, the above findings demonstrate that LMP776 and CPT activate the cytoplasmic cGAS/STING signaling pathway and the expression of immune genes in human HeLa in a manner dependent on STING activation.
We then analyzed SCLC, highlighting that gene activation was lower in SCLC than HeLa cells for both LMP776 and CPT. Actually, STING was expressed in H209, but not in H889 and DMS114 cells, whereas cGAS was expressed in DMS114, but not in H209 and H889 cells. Therefore, the low activation of immune genes in SCLC could be due to reduced STING expression in these cells. Thus, altogether our findings show that immune gene activation induced by LMP776 and CPT is markedly impaired in SCLC cells likely due to a defective STING pathway.
As the findings pointed to the expression of STING and cGAS for immune gene activation in cancer cells, we analyzed expression levels of STING pathway genes in relation to immunological tumor features in lung tumor datasets, focusing on lung adenocarcinoma (LUAD), lung squamous cell carcinoma (LUSC), large cell neuroendocrine lung cancer (LCNEC) and small cell lung cancer (SCLC). We found that STING is significantly downregulated in SCLC and LCNEC compared to LUAD and LUSC types (in which this gene is significantly downregulated compared to normal tissues). This repression is mainly due to a significant hypermethylation in lung tumors, that we observe also in SCLC cell line models. Furthermore, as a result of STING downregulation, we observed a strong negative enrichment for “Response to Interferon Beta” genes in SCLC and LCNEC, that is strongly correlated with STING reduced expression. Overall, our findings indicate that STING expression is generally downregulated in human lung cancers compared to normal tissues and its repression results in a strong inhibition of innate immune response induction.
Taken together our findings show that anticancer TOP1 poisons, CPT and LMP776, increase micronuclei generation with a mechanism involving R-loop accumulation, leading to activation of the cGAS-STING pathway and immune gene expression in HeLa cancer cells. In addition, we show that Top1 poisons can activate both IRF3- and NF-kB-dependent genes, however they cannot in SCLC cancer cells as the cGAS-STING pathway is markedly impaired likely due to several mechanisms including a drastic reduction of STING and/or cGAS expression (Figure 1B).
REFERENCES
- Pommier Y. Topoisomerase I inhibitors: Camptothecins and beyond. Nat Rev Cancer. 6, 789–802 (2006).
- Capranico G, Marinello J, Chillemi G. Type I DNA Topoisomerases. J Med Chem. 60, 2169–92 (2017).
- Pommier Y, Sun Y, Huang SYN, Nitiss JL. Roles of eukaryotic topoisomerases in transcription, replication and genomic stability. Nat Rev Mol Cell Biol. 17, 703–21 (2016).
- Thomas A, Pommier Y. Targeting topoisomerase I in the era of precision medicine. Clin Cancer Res. 25, 6581–9 (2019).
- García-Muse T, Aguilera A. Transcription-replication conflicts: How they occur and how they are resolved. Nat Rev Mol Cell Biol. 17, 553–63 (2016).
- Hamperl S, Bocek MJ, Saldivar JC, Swigut T, Cimprich KA. Transcription-Replication Conflict Orientation Modulates R-Loop Levels and Activates Distinct DNA Damage Responses. Cell. 170, 774-786.e19 (2017).
- Chan KL, Palmai-Pallag T, Ying S, Hickson ID. Replication stress induces sister-chromatid bridging at fragile site loci in mitosis. Nat Cell Biol. 11, 753–60 (2009).
- Liu Y, Nielsen CF, Yao Q, Hickson ID. The origins and processing of ultra fine anaphase DNA bridges. Curr Opin Genet Dev. 26, 1–5 (2014).
- Holmström M, Winters V. Micronucleus induction by camptothecin and amsacrine in bone marrow of male and female CD-1 mice. Mutagenesis. 7, 189–93 (1992).
- MacKenzie KJ, Carroll P, Martin CA, Murina O, Fluteau A, Simpson DJ, et al. CGAS surveillance of micronuclei links genome instability to innate immunity. Nature. 548, 461–5 (2017).
- Harding SM, Benci JL, Irianto J, Discher DE, Minn AJ, Greenberg RA. Mitotic progression following DNA damage enables pattern recognition within micronuclei. Nature. 548, 466–70 (2017).
Follow the Topic
-
British Journal of Cancer

This journal is devoted to publishing cutting edge discovery, translational and clinical cancer research across the broad spectrum of oncology.
Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in