Treatment completion, asparaginase completion, and oncologic outcomes among children, adolescents and young adults with acute lymphoblastic leukemia treated with DFCI Consortium Protocols
Published in General & Internal Medicine
Background
Acute lymphoblastic leukemia (ALL) remains a formidable challenge, particularly for adolescent and young adult (AYA) patients with the disease. Historically, adult patients die from ALL significantly more often than children with a 50% overall survival rate for adult patients, in contrast to a >90% survival in younger children.[i],[ii] Adverse disease biology in adolescents and adults patients accounts for some of the survival discrepancy, but receipt of less intensive (and less effective) treatment is also thought to be a major contributor.
In 2008, the Cancer and Leukemia Group B (CALGB) 10403 study was reported. This prospective single-arm, multi-center, phase II trial applied the Children’s Oncology Group (COG) AALL 0232 ALL regimen to 295 AYA patients aged 17 to 39 years and reported feasibility and an encouraging 73% overall survival at 5 years.[iii] Still, it was notable that only 39% of patients completed protocol assigned treatment. This raised concerns that AYA patients might struggle to complete complex pediatric regimens, either due to regimen toxicity or psychosocial barriers. The treatment completion rate was lower in C10403 than among children (<18 years) and AYA patients (18-30 years) enrolled in the companion pediatric trial AALL 0232 (57% and 74% completed treatment, respectively).
In our study, we aimed to investigate treatment completion rates, reasons for discontinuation, and impact on outcomes among children and AYAs treated on or as per the Dana Farber Cancer Institute (DFCI) Consortium trials.
How Did We Investigate This?
This study analyzed data from four consecutive DFCI ALL Consortium protocols from 2000 to 2021, encompassing 1491 Philadelphia-chromosome negative ALL patients (1159 children aged < 15 years, and 332 AYAs aged 15-50).[iv],[v],[vi],[vii] We included patients enrolled on the four protocols and patients treated “as per” the trials, after enrollment had closed. Treatment completion was defined as receiving the full course of treatment including induction, maintenance, and consolidation. Reasons for not completing treatment were categorized as being due to induction failure, transplantation in first complete remission (CR1), relapse during treatment, death during treatment, and withdrawal from planned treatment. “Withdrawal from planned treatment” included all patients who were taken off study due to clinician decision, personal reasons, social reasons, or toxicity. Our study did not capture additional treatments that patients might have received after withdrawal from planned treatment. DFCI protocols uniquely incorporate 30 weeks of continuous asparaginase in consolidation. Completion of asparaginase was defined as receipt of more than 26 weeks of asparaginase during consolidation based on a previous study.17 This included patients who received alternative asparaginase preparations.
Key Findings
Treatment Completion:
The completion rates for AYA patients (15-50 years) were notably lower (60.8%) compared to children (89.7%) (p < 0.001). Failure to complete therapy was primarily due to more frequent early treatment failure and transplant in CR1 among AYA patients. Importantly, withdrawal from treatment (related to toxicity, personal, or social reasons) was relatively uncommon among AYAs (9.3%) in our cohort.
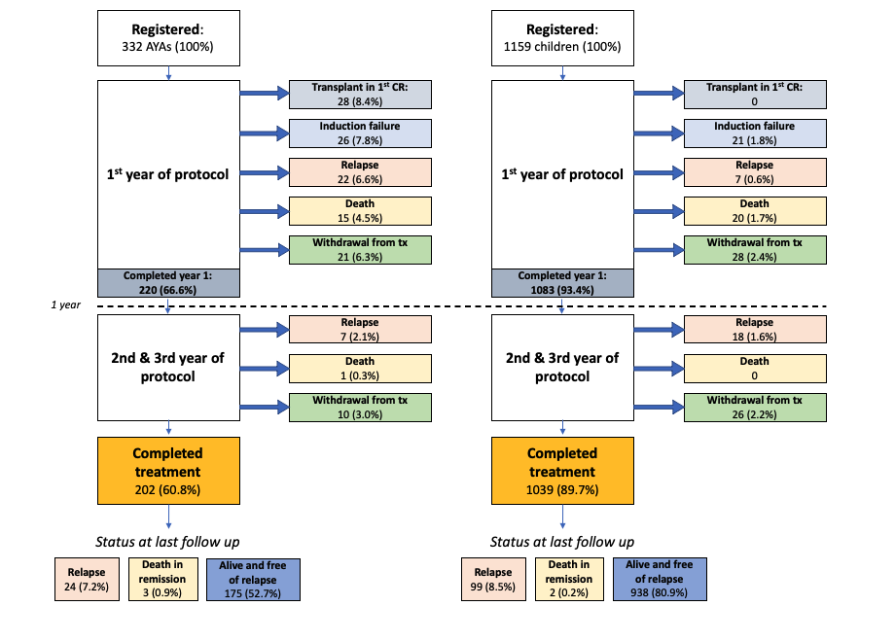
Survival:
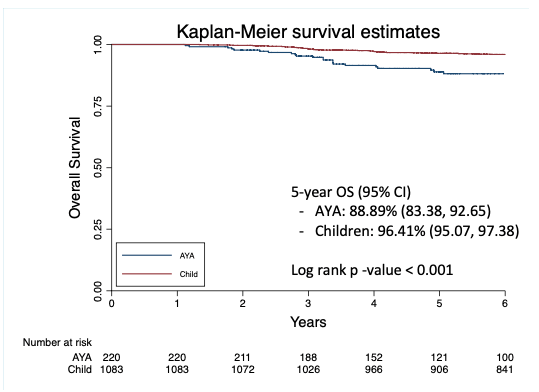
Patients who experienced early treatment failure (induction failure or relapse within 1 year of diagnosis) had poor outcomes with 23.4% overall survival (OS) at 5 years for AYAs and 33.8% for children. Conversely, patients who completed at least one year of protocol assigned treatment had favorable survival, which was statistically better among children: 5-year OS among AYA patients 88.9% versus children 96.4% (p < 0.001) (Figure 2). Notably patients without early treatment failure who withdrew from planned treatment for toxicity, social, or unknown reasons also had favorable long-term outcomes: 5-year OS among AYA patients 87.8% and among children 94.2% (p = 0.2). Our study did not record post-protocol treatment for these patients.
Asparaginase Completion:
Asparaginase, a crucial component of ALL treatment, presented its own set of challenges. AYA patients completed the majority of prescribed asparaginase less frequently (79.1%) than children (89.6%) (p < 0.001), primarily due to increased toxicity. The OS at 5 years among 1303 patients who completed at least 1 year of treatment was higher for those who completed asparaginase (95.8%) than those who did not (91.0%, p = 0.027). In a multivariable analysis, asparaginase remained significantly associated with OS: for every week of consolidation asparaginase received, patients had a 3% lower hazard of death.
Take-Home Message
In this large retrospective study that included both children and AYAs with ALL treated on pediatric inspired protocols at DFCI, we found that AYAs completed treatment at significantly lower rates than children. Notably, this difference was driven primarily by treatment failure, which is likely related to disease biology, and not patient unwillingness or inability to complete treatment.
AYAs in our study completed treatment at substantially higher rates (60.8% overall, 72.9% in those without early biologic treatment failure or transplant in first CR) than those previously reported by the CALGB 10403 investigators (39%). This difference appears to be driven by more patients in the CALGB cohort withdrawing from study for toxicity, social/personal or unknown reasons compared to DFCI Consortium patients: 27% of patients in CALGB 10403 vs. 9% of AYA patients in our study fall in this category. Rates of induction failure, death while on treatment, and relapse while on treatment are similar between the two studies.
Given that most of the poor outcomes among AYA patients in our study were driven by early treatment failure, ongoing efforts to identify high risk patients early and allocate them to novel approaches and more intensive treatment, including allogeneic stem cell transplant, will be crucial to improve the outcomes of this population. For patients without early treatment failure, the need for novel agents in consolidation, which has been demonstrated to be beneficial in older patients, is less certain given extremely low risk of relapse. Ongoing research in optimizing delivery of asparaginase in AYA patients and reducing the incidence of toxicities resulting in asparaginase truncation will also help further improve outcomes in responding patients with the hope of closing the gap between children and AYA patients in the future. Lastly, further research is needed to determine the optimal treatment of patients who cannot tolerate asparaginase and whether novel approaches, such as the incorporation of blinatumomab consolidation, can be used to improve outcomes.[viii]
[i] Vrooman LM, Blonquist TM, Stevenson KE, Supko JG, Hunt SK, Cronholm SM, et al. Efficacy and Toxicity of Pegaspargase and Calaspargase Pegol in Childhood Acute Lymphoblastic Leukemia: Results of DFCI 11-001. J Clin Oncol. 2021 Nov 1;39(31):3496-3505. doi: 10.1200/JCO.20.03692. Epub 2021 Jul 6. PMID: 34228505.
[ii] Patel B, Rai L, Buck G, Richards SM, Mortuza Y, Mitchell W, et al. Minimal residual disease is a significant predictor of treatment failure in non T-lineage adult acute lymphoblastic leukaemia: final results of the international trial UKALL XII/ECOG2993. Br J Haematol. 2010 Jan;148(1):80-9. doi: 10.1111/j.1365-2141.2009.07941.x. Epub 2009 Oct 26. PMID: 19863538.
[iii] Stock W, Luger SM, Advani AS, Yin J, Harvey RC, Mullighan CG, et al. A pediatric regimen for older adolescents and young adults with acute lymphoblastic leukemia: results of CALGB 10403. Blood. 2019 Apr 4;133(14):1548-1559.
[iv] Barry E, DeAngelo DJ, Neuberg D, Stevenson K, Loh ML, Asselin BL, et al. Favorable outcome for adolescents with acute lymphoblastic leukemia treated on Dana-Farber Cancer Institute Acute Lymphoblastic Leukemia Consortium Protocols. J Clin Oncol. 2007 Mar 1;25(7):813-9. doi: 10.1200/JCO.2006.08.6397. PMID: 17327603.
[v] Vrooman LM, Blonquist TM, Harris MH, Stevenson KE, Place AE, Hunt SK, et al. Refining risk classification in childhood B acute lymphoblastic leukemia: results of DFCI ALL Consortium Protocol 05-001. Blood Adv. 2018 Jun 26;2(12):1449-1458. doi: 10.1182/bloodadvances.2018016584. PMID: 29941458; PMCID: PMC6020806.
[vi] Vrooman LM, Stevenson KE, Supko JG, O'Brien J, Dahlberg SE, Asselin BL, et al. Postinduction dexamethasone and individualized dosing of Escherichia Coli L-asparaginase each improve outcome of children and adolescents with newly diagnosed acute lymphoblastic leukemia: results from a randomized study--Dana-Farber Cancer Institute ALL Consortium Protocol 00-01. J Clin Oncol. 2013;31(9):1202-1210.
[vii] DeAngelo D, Dahlberg S, Silverman L, Couban S, Amrein P, Seftel M, et al. A Multicenter Phase II Study Using a Dose Intensified Pediatric Regimen in Adults with Untreated Acute Lymphoblastic Leukemia. Blood. 2015;110. 587-587.
[viii] Litzow MR, Sun Z, Paletta E, Mattison R, Lazarus H, Rowe J, et al. Consolidation therapy with blinatumomab improves overall survival in newly diagnosed adult patients with B-lineage acute lymphoblastic leukemia in measurable residual disease negative remission: Results from the ECOG-ACRIN E1910 randomized phase III National Cooperative Clinical Trials Network trial. Blood. 2022 140 (Supplement 2): LBA-1.
Follow the Topic
-
Leukemia

This journal publishes high quality, peer reviewed research that covers all aspects of the research and treatment of leukemia and allied diseases. Topics of interest include oncogenes, growth factors, stem cells, leukemia genomics, cell cycle, signal transduction and molecular targets for therapy.
Your space to connect: The Cancer in understudied populations Hub
A new Communities’ space to connect, collaborate, and explore research on Cancers, Race and Ethnicity Studies and Mortality and Longevity!
Continue reading announcement

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in