Zebrafish imaging reveals TP53 mutation switching oncogene-induced senescence from suppressor to driver in primary tumorigenesis
Published in Cancer

Most tumors are believed to arise through oncogenic cell generation followed by additional mutations. However, it remains unclear how a newly emerged oncogenic cell primes tumorigenesis through the acquisition of additional mutations. In addition, the interaction between a newly generated oncogenic cell and surrounding normal cells in the early stages of tumorigenesis has not been well studied.
Recent cancer studies have revealed that oncogenic cells undergo cellular senescence, which plays both tumor-suppressive and tumor-promoting roles depending on the cellular context. Senescence plays tumor-suppressive roles by inducing irreversible cell cycle arrest or activating immune cell-mediated cell elimination, whereas senescent cells can also mediate tumorigenic effects by secreting a variety of inflammatory cytokines and growth factors, referred to as the senescence-associated secretory phenotype (SASP). However, the roles of SASP in primary tumorigenesis are unknown. In addition, what decides the tumor-suppressive or tumorigenic effects of cellular senescence in oncogenic cells is also unclear.
In the present study (Haraoka et al., Nat Commun. 2022), we visualized the oncogenic cell behavior by using zebrafish skin as a model of the human epithelia and discovered the following previously unrecognized mechanisms in primary tumorigenesis:

(1) A newly emerged oncogenic cell with the RasG12V mutation becomes senescent through communication with neighboring cells and is eliminated from the zebrafish epithelia.
(2) RasG12V cell elimination is prevented by the addition of a TP53 gain-of-function mutation (TP53R175H) to the RasG12V cell.
(3) The surviving RasG12V-TP53R175H double mutant cells also senesce and then secrete SASP factors, which stimulate the proliferation and senescence of neighboring cells.
(4) Secondary senesced neighbors also secrete SASP factors, thereby propagating cellular senescence and stimulating aberrant cell proliferation in the neighborhood, resulting in the formation of a heterogeneous tumor.
(5) RasG12V cells efficiently form heterogeneous tumors through secondary senescence in damaged or TP53-activated epithelia, but not in healthy epithelia.Taken together, our study reveals that an additional TP53 mutation switches oncogene-induced senescence from a tumor suppressor to driver, thereby priming tumorigenesis. Previous studies have revealed two tumor-suppressive mechanisms of cellular senescence (Collado and Serrano, Nat. Rev. Cancer 2010; Gorgoulis et al., Cell 2019). The first is the induction of irreversible cell cycle arrest in oncogenic cells. The second is the stimulation of immune cell-mediated senescent cell elimination. In this study, we discovered immune cell-independent oncogenic cell elimination as a third anti-tumor mechanism of cellular senescence.
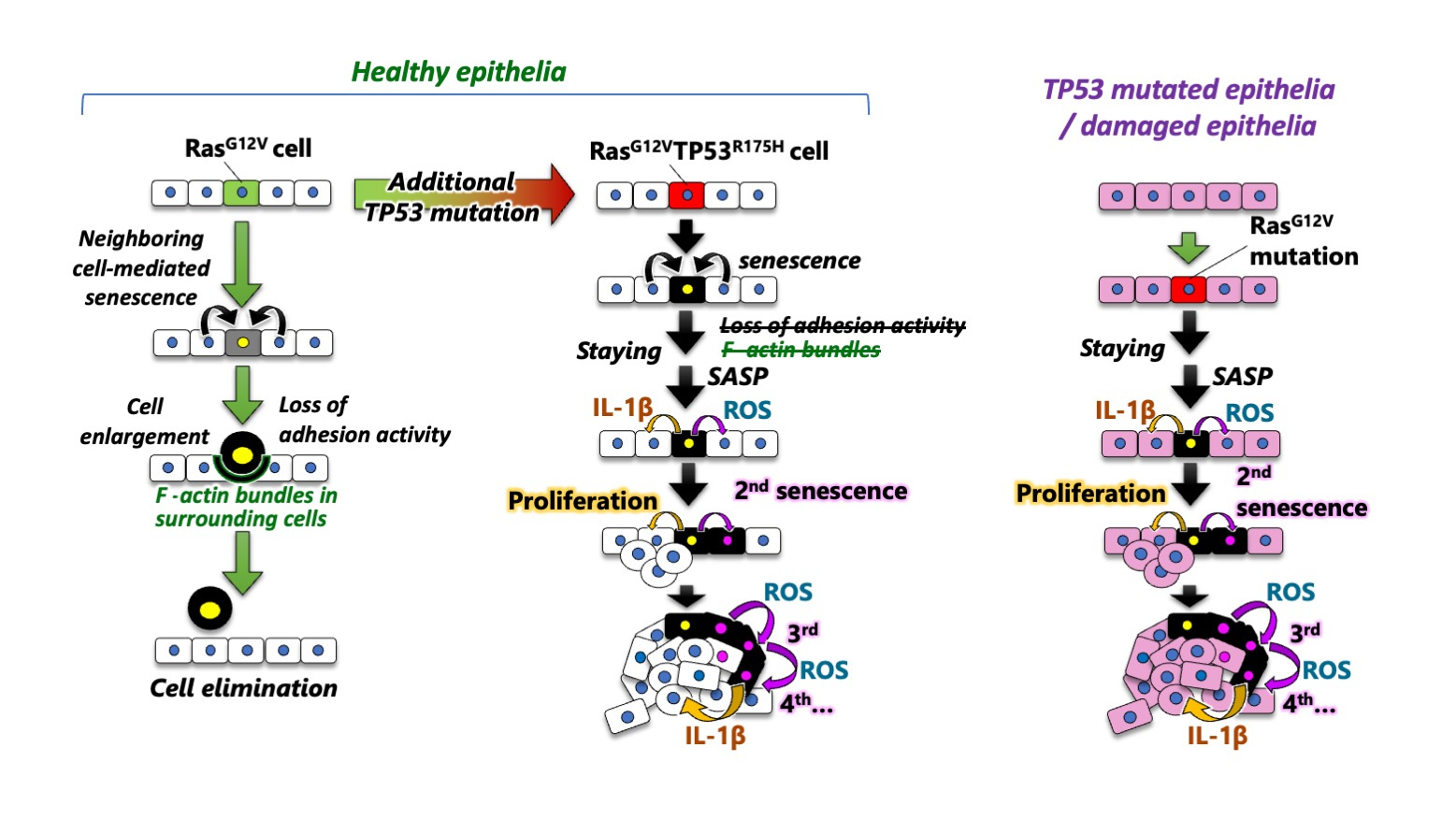 Our work also sheds light on a previously elusive interaction between oncogenic cells and their surrounding cells during the early stages of cancer development. Newly emerged oncogenic cells with single Ras mutations are eliminated through communication with neighboring cells in healthy epithelia, whereas Ras-TP53 double mutant cells survive and induce proliferation and secondary senescence in surrounding cells. The predominance of oncogenic cells over their neighboring cells changes during primary tumorigenesis.
Our work also sheds light on a previously elusive interaction between oncogenic cells and their surrounding cells during the early stages of cancer development. Newly emerged oncogenic cells with single Ras mutations are eliminated through communication with neighboring cells in healthy epithelia, whereas Ras-TP53 double mutant cells survive and induce proliferation and secondary senescence in surrounding cells. The predominance of oncogenic cells over their neighboring cells changes during primary tumorigenesis.
Thus, by using zebrafish imaging analyses, we succeeded in revealing previously unidentified oncogenic cell behaviors, which may control the initial step of human tumorigenesis.
REFERENCES:
Haraoka, Akieda, Nagai, Mogi, and Ishitani, Zebrafish imaging reveals TP53 mutation switching oncogene-induced senescence from suppressor to driver in primary tumorigenesis. Nat Commun. 2022 March; 13: 1417 doi: 10.1038/s41467-022-29061-6
Collado and Serrano, Senescence in tumours: evidence from mice and humans. Nat Rev Cancer. 2010 Jan;10(1):51-7. doi: 10.1038/nrc2772.
Gorgoulis et al., Cellular Senescence: Defining a Path Forward. Cell 2019 Oct 31;179(4):813-827. doi: 10.1016/j.cell.2019.10.005.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in