9.44% Photo-charging Efficiency Solar Redox Flow Cell via Single-photon Device
Published in Electrical & Electronic Engineering

An associated article published in Communications Materials can be found here:https://www.nature.com/article... (DOI: 10.1038/s43246-020-0020-7)
The combination of a photo-device with an electrochemical system, such as photoelectrochemical (PEC) water splitting, has attracted much attention for the last decades because the direct conversion of solar energy into chemical is an attractive route to produce clean fuel. However, we are currently at an interesting juncture where its days as a prospective renewable energy source are fading due to its sluggish reaction kinetics and poor technological readiness.1,2
Alternatively, solar rechargeable redox flow batteries (SRFBs) have drawn considerable interest from energy storage researchers. Generally, redox flow batteries (RFBs) present facile kinetics, which is several orders of magnitudes faster than that for the water-splitting processes. The simple architectural concept of the SRFB is also its technological readiness. As shown in figure below (Fig. 1), a combination of an RFB and a PEC photo-charging component is used as a means of simultaneous storage of solar energy into chemicals, which can be readily utilized to generate electricity.

Despite these advantages, recent SRFB studies are confronted with slow kinetics, which is opposite to the trend in RFB studies. The unforeseen poor reaction kinetics are attributed to an inadvertent application without careful consideration of solid/liquid interfaces, which may be different from typical catalytic reactions. In practice, we have fabricated various c-Si photoelectrodes to demonstrate a dependency of ferri-/ferrocyanide redox reaction reactivity on the type of conducting materials (carbon and Pt) and device architecture.
During the last two years, we have successfully demonstrated various SRFB models,1,3–5 but what was particularly difficult in this work was the identification of the carbon conducting layers’ morphology. Above all things, the carbon tends to aggregate forming nanoparticles rather than covering the surface with a conformal thin film, and this hampered precise measurement of carbon thickness. In order to solve this, we have conducted multiple analyses using both AFM (atomic force microscopy operated by A. Venugopal; see Fig. 2a and b) and sub-nanometer resolution profilometer, which could serve as a complementary one to the other.
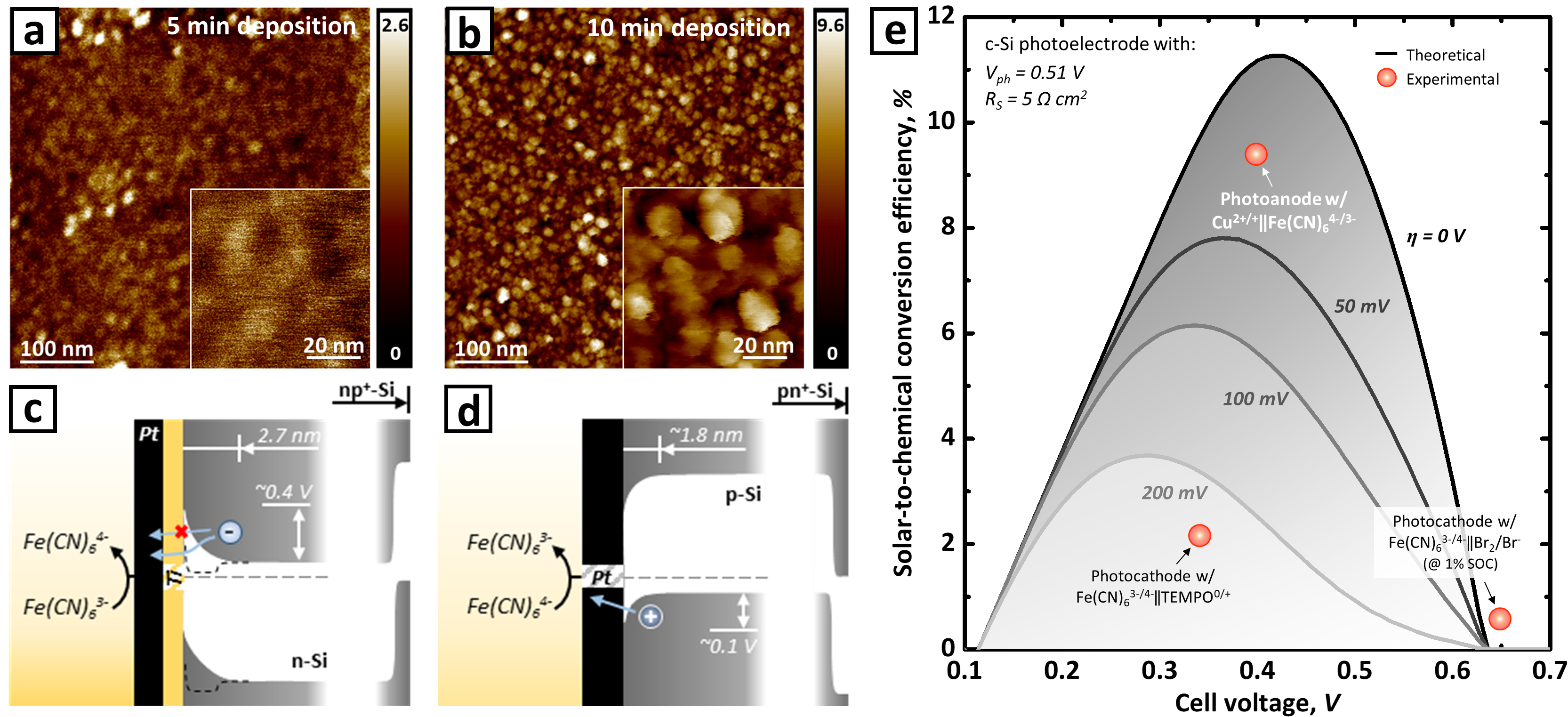
Another challenge was the energetic barrier at the interface between the photoelectrode and the conducting catalyst layer. Forming a feasible carrier pathway between the high work-function material and n-type Si has been one of the long-standing challenges in the semiconductor field (Fig. 2c). We simply avoided this question by choosing an inverse device structure with a p-type surface, and this led to only a shallow Schottky barrier that positively charged carriers could tunnel through (Fig. 2d). Our efforts to design an appropriate surface/interface resulted in the highest PEC charging efficiency (9.44 %; Fig. 2e) for the SRFBs with a single-photon device reported thus far. Based on our results, we have started believing that our single-junction system might eventually be able to deservedly compete with the state-of-the-art multi-junction-based SRFB system demonstrated to date.6,7
In the background of our achievement, besides the authors’ scientific contribution, there have been strong administrative and financial supports. Particularly, Prof. Bernard Dam, section head of the Materials for Energy Conversion and Storage (MECS) group, has provided a various form of academic advice with an appropriate workspace for the main author during his guest research stay. The library of the TU Delft also provided financial support for this publication with an open-access fund. The authors also thank Dr. Kristina Wedege (Aarhus University; currently at McKinsey & Company) for assistance with TEMPO-sulfate synthesis. The support of silicon substrates by the Dr. Choongman Moon (Technical University of Denmark) is also acknowledged.

1. Bae, D. et al. Unravelling the practical solar charging performance limits of redox flow battery based on the single photon device system. Sustain. Energy Fuels 3, 2399–2408 (2019).
2. Bae, D. et al. Strategies for stable water splitting: Via protected photoelectrodes. Chem. Soc. Rev. 46, 1933–1954 (2017).
3. Wedege, K. et al. Unbiased, complete solar charging of a neutral flow battery by a single Si photocathode. RSC Adv. 8, 6331–6340 (2018).
4. Wedege, K. et al. Solar Redox Flow Batteries with Organic Redox Couples in Aqueous Electrolytes : A mini-review. J. Phys. Chem. C 122, 25729–25740 (2018).
5. Bae, D. et al. Tailored energy level alignment at MoOX/GaP interface for solar-driven redox flow battery application. J. Chem. Phys. 152, 124710 (2020).
6. Li, W. et al. 14.1% Efficient Monolithically Integrated Solar Flow Battery. Chem 4, 2644–2657 (2018).
7. Urbain, F. et al. Solar vanadium redox-flow battery powered by thin-film silicon photovoltaics for efficient photoelectrochemical energy storage. J. Phys. D. Appl. Phys. 52, 044001 (2019).
Follow the Topic
-
Communications Materials

A selective open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of materials science.
Related Collections
With Collections, you can get published faster and increase your visibility.
Advanced characterizations of high-entropy materials
Publishing Model: Open Access
Deadline: Mar 31, 2026
Multifunctional hydrogels
Publishing Model: Open Access
Deadline: Feb 28, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in