A genetically attenuated malaria vaccine candidate with superior anti-malarial immunity
Published in Microbiology and Biomedical Research

Despite decades of research, malaria remains one of the leading public health problems in the world, affecting an estimated 249 million cases and more than 608,000 deaths globally. The WHO has already approved two subunit vaccines, RTS,S, and R21/Matrix-M. However, their long-lasting efficacy and practical applications in endemic areas remain of concern. One of the practical approaches is a genetically attenuated parasite (GAP)-based vaccination that is arrested during late liver stage development. This study created a late liver stage arrested GAP (LA-GAP) based on the double deletion of the Scd/Scot1 genes in malaria parasite P. berghei, which can induce superior preerythrocytic and stage transcending immunity 1.
Why the LA-GAP is superior for vaccination
The whole sporozoite (WSpz) vaccination strategy offers a promising alternative to existing subunit vaccines, as it involves a diverse antigen repertoire. This WSpz vaccination strategy is based on the attenuation of sporozoites by different mechanisms. Parasites may be attenuated by irradiation (radiation attenuated parasites, RAS) or genetic manipulation (GAP) or live sporozoite immunization along with antimalarials (chemo-prophylaxis and sporozoites, CPS). RAS does not express late liver-stage antigens, and CPS- poses safety risks. GAPs may confer potential advantages over others, as they are intrinsically attenuated and consistent from batch to batch. Early liver-stage arresting replication-deficient (EARD) are first-generation GAPs that are arrested in trophozoites or in schizogony stages after invading the liver. Late liver stage attenuated replication-competent (LARC) GAPs complete intrahepatocytic development, increasing the parasite biomass and antigen repertoire before arrest. Compared with EARD, LARC confers superior protection. Overall, LA-GAP is considered the best and is currently under development.
The discovery of a safe Scd/Scot1 LA-GAP
We found that the deletion of Scd (Stearoyl-Co-A Δ9- desaturase) 2 and Scot1 (sporozoite conserved orthologous transcripts 1) 3 in Plasmodium berghei causes attenuation of the parasites in the late live stage, and the KO parasites fail to initiate blood-stage infection after up to 50,000 intravenous inoculations of sporozoites. Immunization with both types of KO sporozoites conferred complete protection against the infectious sporozoite challenge. However, we observed occasional breakthrough infections in both cases with higher doses of sporozoites. To overcome this breakthrough infection, a double gene deletion mutant parasite (Scd/Scot1 KO)was generated.
The effect of dual gene deletion on parasite development
Dual gene deletion did not affect parasite development in the blood or mosquito stages. Mutant sporozoites invade the liver and grow normally but fail to mature into hepatic merozoites. The branching of the apicoplast was impaired in the Scd/Scot1-KO liver stage. The parasites never transition to blood, even after a high dose of sporozoite infection.
Superior antimalarial immunity by Scd/Scot1 GAP
C57BL/6 mice receiving two or three doses of sporozoites were protected against infectious sporozoite challenge. Compared with two immunizations, immunization with three doses provided better and longer-lasting protection. Immunization with LA-GAP elicits cellular and humoral immune responses. An increased number of CD8+ T cells in immunized mice seems to play a crucial role in parasite clearance. Memory subsets of CD8+ T cells also increased in a dose-dependent manner, eliciting long-lasting protection in LA-GAP-immunized mice. To prove the superiority of LA-GAPs, we compared the immune response with that of EA-GAPs, which arrest during the early-mid-liver stage of development 4. Compared with EA-GAPs, LA-GAPs induce greater and broader CD8+ T-cell responses and elicit stage-transcending immunity that provides superior protection in C57BL/6 mice. Antibodies in LAGAP-immunized mice recognize all parasite life stages, whereas EAGAP immune serum recognizes only the sporozoite and liver stages.
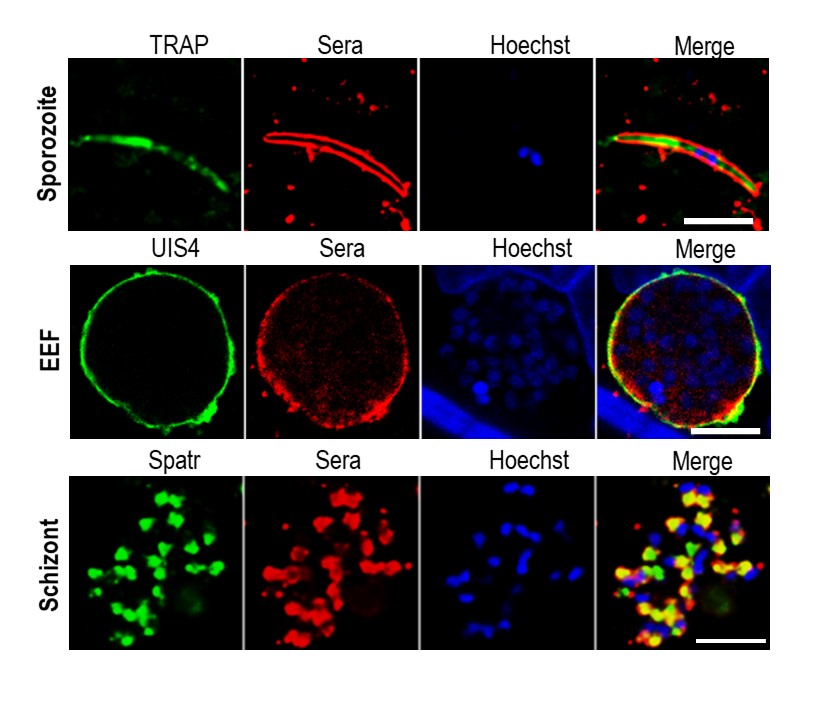
mouse sera.
Looking Ahead
Different preclinical and clinical trials underscore the potential of LA-GAP-based vaccines. The discovery of Scd/Scot1 KO parasites as safe LA-GAPs illuminates the path of malaria vaccine development. Ultimately, the future entails the generation of Scd/Scot1 GAP in the human malaria parasite P. falciparum for immunizing humans.
References:
- Mishra, A., Paul, P., Srivastava, M. & Mishra, S. A Plasmodium late liver stage arresting GAP provides superior protection in mice. npj Vaccines 9, 1–15 (2024).
- Narwal, S. K. et al. Stearoyl-CoA desaturase regulates organelle biogenesis and hepatic merozoite formation in Plasmodium berghei. Mol. Microbiol. (2024) doi:10.1111/mmi.15246.
- Ghosh, A. et al. A Micronemal Protein, Scot1, Is Essential for Apicoplast Biogenesis and Liver Stage Development in Plasmodium berghei. ACS Infect. Dis. 10, 3013–3025 (2024).
- Al-Nihmi, F. M. A. et al. A Novel and Conserved Plasmodium Sporozoite Membrane Protein SPELD is Required for Maturation of Exo-erythrocytic Forms. Sci. Rep. 7, 40407 (2017).
Follow the Topic
-
npj Vaccines

A multidisciplinary journal that is dedicated to publishing the finest and high-quality research and development on human and veterinary vaccines.
Related Collections
With Collections, you can get published faster and increase your visibility.
Lipid nanoparticle (LNP)-adjuvanted vaccines
Publishing Model: Open Access
Deadline: Feb 19, 2026
Therapeutic HPV vaccines
Publishing Model: Open Access
Deadline: Jun 30, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in