A new automated, multiplex approach to studying shear stress mechanotransduction of circulating cells
Published in Bioengineering & Biotechnology, Materials, and Cell & Molecular Biology
Cells that circulate in the bloodstream, including leukocytes and rare tumor cells, respond to fluid forces in profound ways that have important implications in metastatic cancer and diseases of the immune system. My laboratory became interested in such mechanotransduction problems years ago, finding that physiological shear stress can modulate the sensitivity of primary human neutrophils to activation via various chemical stimuli (Mitchell and King, 2012; Mitchell et al., 2014a). At that time, our device of choice for subjecting suspended cells to a precise and uniform shear stress sustained for minutes to hours was the cone-and-plate viscometer, an instrument well known to chemical engineers for measuring the viscosity of various industrial liquids. Using this system we also found that shear stress greatly sensitizes cancer cells to undergo apoptosis in response to TRAIL ligand (Mitchell and King, 2013), and later determined that this is due to the shear-induced activation of the Piezo1 ion channel, leading to calcium influx (Hope et al., 2019).
Of course, fluid flow in the vascular system isn't always laminar and steady as in the cone-and-plate viscometer, it varies widely and includes brief moments of extremely high shear stress magnitude, such as when cells pass through the heart. To model those brief pulses of intense, destructive shear stress, we adopted a simple flow device consisting of the injection of cell suspensions through a syringe needle using a syringe pump, thus subjecting cells to millisecond pulses of stress 1000X as strong as the forces found in the microcirculation. Using this system we found that breast cancer cells are almost as good as blood cells as surviving such forces (Mitchell et al., 2015), and later showed that some prostate cancer cells are able to resist these destructive forces by rapidly repairing the temporary membrane pores that are created by the flow (Hope et al., 2021).
The responses of CTCs to fluid forces, and their ability to survive the extreme, destructive forces these cells are exposed to in the circulation, are of paramount importance to understanding and controlling metastatic cancer. Recent evidence suggests that CTC clusters are one of the most compelling and essential of biomarkers, indicative of the worst clinical outcomes in metastatic cancer. Of course, once you start considering clusters of different subclones of cancer cells, their association with various stromal and immune cells as found in the blood of metastatic cancer patients (Ortiz-Otero et al., 2020a,b), the parameter space one must experimentally address quickly expands to an unmanageable degree. Further motivating this exciting direction of mechanobiology research, recent research from our group has revealed that various immune cells, including T cells (Hope et al., 2022) and dendritic cells (Dombroski et al., 2024), show profound enhancement in cellular activation in response to exposure to physiological levels of fluid shear stress. For all of these reasons, we set out to develop a higher throughput, semi-automated method for subjecting different samples of suspended cells (e.g., cancer or immune cells) to a range of shear stress stimuli, preferably in a form amenable to downstream analysis and easy for nonspecialists to adopt.
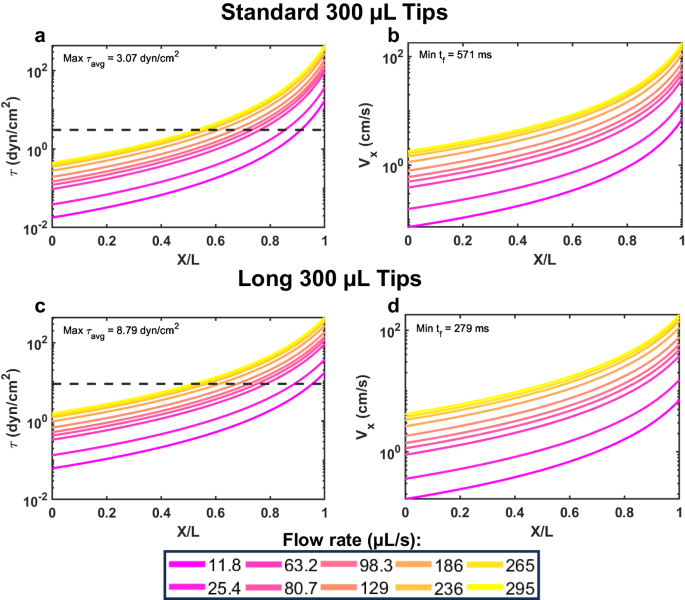
That brings us to our latest publication, in Communications Biology. In it, we present a new multiplex fluidic system for subjecting up to 96 samples at once (or 24, or 12, or 8, etc.) to pre-programmed shear stress signals that can exceed 10,000 shearing cycles, lasting several hours in total duration. Our system is based on a commercially available programmable multipipetter made by Allegra, called the VIAFLO96. The shearing process involves dipping the pipette tips into the sample wells, withdrawing the sample volumes into the pipette tips, then injecting the contents back into each well, what is termed a "shearing cycle". The Allegra system is compatible with three different pipette tip sizes, operates at 10 different flow rates, and many different patterns of shearing cycles can be programmed, enabling lab automation. We provide many useful "VIALINK" programs with our paper, along with some analysis programs implemented in Matlab. To achieve even higher shear stress magnitudes than can be produced using the standard pipette tips, we provide a protocol to equip the instrument with up to 96 syringe needle tips, in a form that can be autoclaved and reused many times. The choice of designing our approach around the (96) multiwell plate platform was to facilitate downstream analysis in plate readers, flow cytometers, etc., that can process multiwell plates. Detailed protocols and parts lists to adopt this approach are provided with the paper (see, for instance, Figure 1), as well as several different example data sets demonstrating mechanotransduction responses of cancer cells and primary murine dendritic cells.
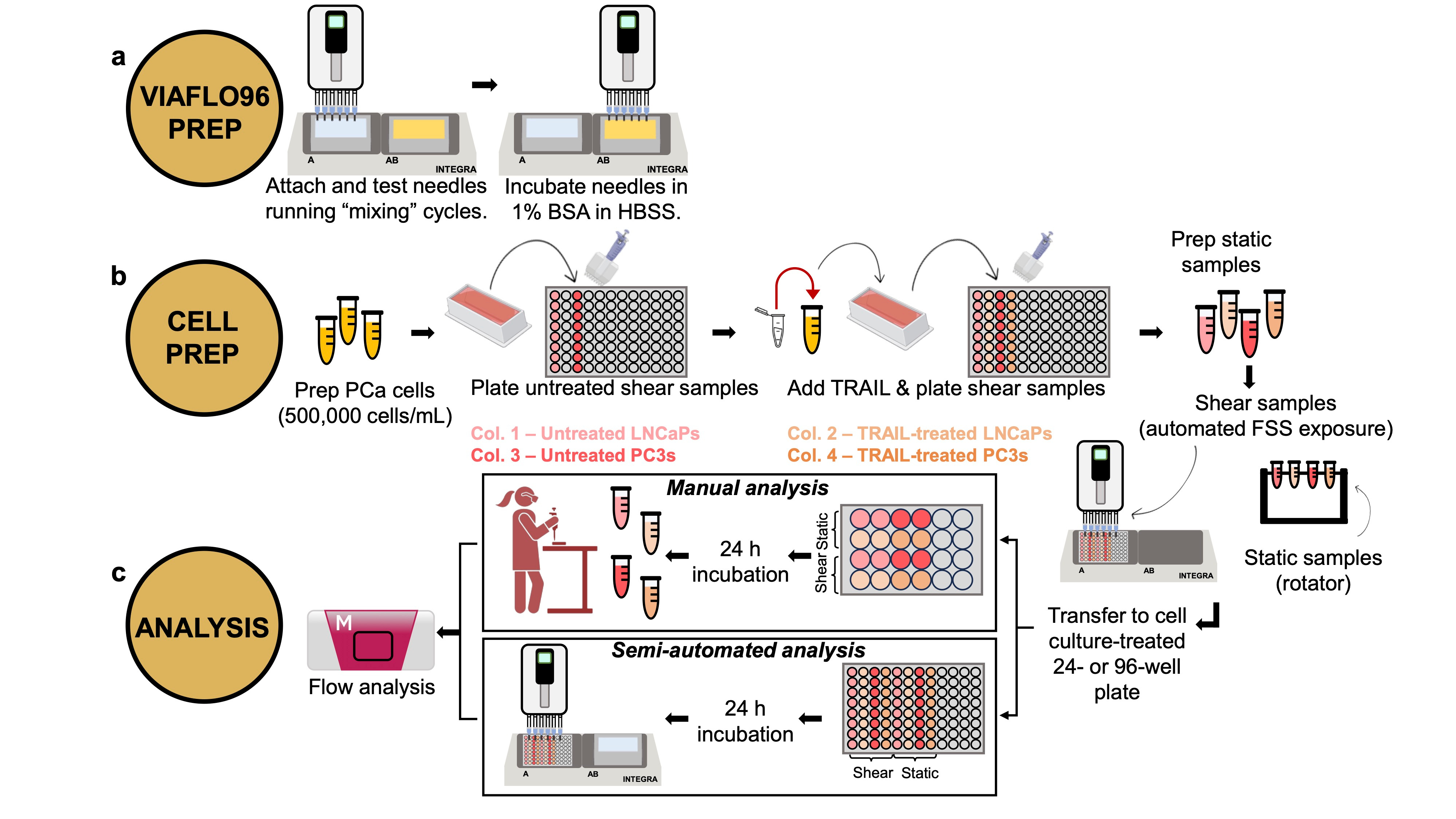
We hope that this new approach for performing higher throughput mechanobiology research on suspended cells will be useful to the research community. The development of this system was supported by the Tissue Engineering Collaborative program of the U.S. National Cancer Institute (Grant No. CA256054), and this publication is a key deliverable of that project. This methodology does not require any specialized knowledge of materials science or microfluidics, and does not require access to a machine shop or clean room facility. We hope that biologists and engineers alike will embrace this new tool for mechanobiology research, and we look forward to seeing new discoveries and breakthroughs in the future!
References:
Dombroski JA, Rowland SJ, Fabiano AR, Knoblauch SV, Hope JM, King MR. 2023. Fluid shear stress enhances dendritic cell activation. Immunobiology 228:152744.
Hope JM, Bersi MR, Dombroski JA, Clinch AB, Pereles RS, Merryman WD, King MR. 2021. Circulating prostate cancer cells have differential resistance to fluid shear stress-induced cell death. J. Cell Sci. 134:jcs251470.
Hope JM, Dombroski JA, Pereles RS, Lopez-Cavestany M, Greenlee JD, Schwager SC, Reinhart-King CA, King MR. 2022. Fluid shear stress enhances T cell activation through Piezo1. BMC Biol. 20:61.
Hope JM, Lopez-Cavestany M, Wang W, Reinhart-King CA, King MR. 2019. Activation of Piezo1 sensitizes cells to TRAIL-mediated apoptosis through mitochondrial outer membrane permeability. Cell Death Dis. 10:837.
Mitchell MJ, Denais C, Chan MJ, Wang Z, Lammerding J, King MR. 2015. Lamin A/C deficiency reduces circulating tumor cell resistance to fluid shear stress. Am. J. Physiol. Cell Physiol. 309:C736-46.
Mitchell MJ, King MR. 2012. Shear-induced resistance to neutrophil activation via the formyl peptide receptor. Biophys. J. 102:1804-14.
Mitchell MJ, King MR. 2013. Fluid shear stress sensitizes cancer cells to receptor-mediated apoptosis via trimeric death receptors. New J. Phys. 15:015008.
Mitchell MJ, Lin KS, King MR. 2014a. Fluid shear stress increases neutrophil activation via platelet-activating factor. Biophys. J. 106:2243-53.
Ortiz-Otero N, Clinch AB, Hope JM, Wang W, Reinhart-King CA, King MR. 2020a. Cancer associated fibroblasts confer shear resistance to circulating tumor cells during prostate cancer metastatic progression. Oncotarget 11:1037-50.
Ortiz-Otero N, Marshall JR, Lash B, King MR. 2020b. Chemotherapy-induced release of circulating-tumor cells into the bloodstream in collective migration units with cancer-associated fibroblasts in metastatic cancer patients. BMC Cancer 20:873.
Follow the Topic
-
Communications Biology

An open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of the biological sciences, representing significant advances and bringing new biological insight to a specialized area of research.
Related Collections
With Collections, you can get published faster and increase your visibility.
Forces in Cell Biology
Publishing Model: Open Access
Deadline: Apr 30, 2026
Signalling Pathways of Innate Immunity
Publishing Model: Hybrid
Deadline: May 31, 2026

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in