A Synthesis Strategy for Tetracyclic Terpenoids Leads to Agonists of the Estrogen Receptor Beta
Published in Chemistry

Explore the Research
s41467-019-10415-6.pdf?utm_campaign=related_content&utm_source=HEALTH&utm_medium=communities
Overall: Our manuscript has emerged from a collaborative program that began with questions regarding basic organic chemistry and natural product synthesis, being fueled by the desire to establish a chemical technology platform capable of facilitating the search for natural product-inspired agents of medicinal value. Efforts from my laboratory in the Department of Chemistry at Dartmouth College were focused on establishing the organic chemistry and led to the initial discovery of a potent and selective agonist of a clinically relevant nuclear hormone receptor [the estrogen receptor beta (ERb)]. Professor Thomas Burris at the St. Louis College of Pharmacy and Washington University School of Medicine (and a former colleague of mine at The Scripps Research Institute) established that our lead small molecules were agonists of ERb in prostate cancer cells (DU-145). With an appreciation that selective agonism of ERb may be useful as a strategy to treat a variety of cancers in addition to prostate cancer, Professor Arti Gaur and her team in the Department of Neurology at the Geisel School of Medicine and the Norris Cotton Cancer Center discovered that our new compositions of matter have potent and selective toxicity towards glioblastoma (vs. human neural stem cells and human astrocytes) and are exciting leads for the development of first-in-class chemotherapeutics.
Some Background: Natural products continue to play a significant role in chemical biology and medicine, and the desire to explore compositions of matter related to or inspired by them has been a driving force for the development of synthetic organic chemistry as a discipline (new reactions and synthesis strategies). Our broad objective has been advancing the science of organic synthesis with this "selective pressure" in mind, and since the early 2000s we have established dozens of new stereoselective reactions in organic chemistry, several of which have played prominent roles in natural product and function-oriented synthesis programs. Recently, our attention has been directed toward a large class of medicinally relevant natural products that we collectively refer to as "tetracyclic terpenoids." Examples of molecules within this class include limonoids, lanostanes, euphanes, tirucallanes, cucurbitanes, and steroid hormones (i.e., estranes, androstanes, pregnanes), among others, and the class as a whole is well understood to be the most successful among natural product-inspired drugs in the pharmaceutical industry (>100 FDA-approved drugs are known from the group).
While the history of organic chemistry is replete with contributions that target de novo synthesis of tetracyclic terpenoid natural products, it remains the case that "total synthesis" of these molecules often remains quite labor intensive and inefficient (i.e., established chemical synthesis pathways can be cumbersome to employ as a foundation to drug discovery and development). While “semi-synthesis” has enabled this area of pharmaceutical science, and remains of interest in modern natural product synthesis pursuits, this approach is not without substantial limitations that include generally being constrained to exploration within a single enantiomeric series. Because drug-like properties are largely independent of absolute stereochemistry, simply considering this single limitation led us to conclude that vast regions of pharmaceutically privileged regions of chemical space have been under-explored for decades. In fact, 100% of the FDA-approved therapeutics from this natural product-inspired class are of the “natural” enantiomeric configuration (a fact that we believe is due, in part, to the style of synthesis typically embraced to explore the medicinal value of such natural product-inspired compositions of matter). So, we began contemplating the invention and development of new organic chemistry capable of enabling uncharted medicinal exploration in this broad area of natural product-inspired science. Our first advance in this area was focused on the synthesis of partially aromatic tetracycles and was published in early 2018, with the current work offering conceptually unique chemistry that addresses a wider variety of problems (including the establishment of numerous quaternary center).
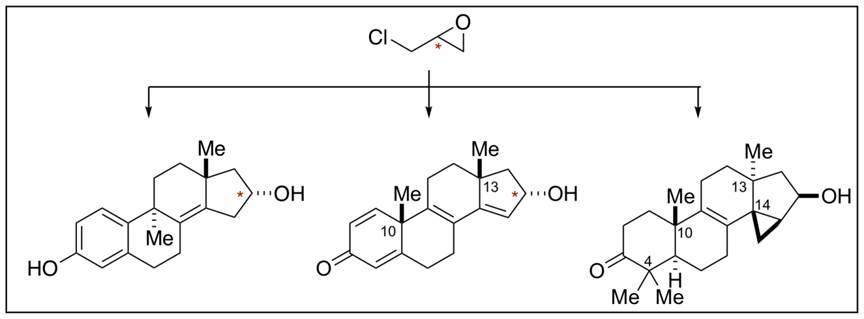
The Chemical Advance: A synthesis strategy for the asymmetric construction of tetracyclic terpenoid motifs bearing characteristic quaternary centers at C9, C10, C13, and even C14 (steroid numbering) is described. The approach was made possible first by the power of one of our recently developed metallacycle-mediated annulative cross-coupling reactions that deliver a hydrindane motif bearing a “C-ring” endo diene, and then through the design and development of two additional chemical transformations: (1) a double asymmetric tandem protodesilylation and Friedel–Crafts cyclization to forge the terpenoid “B” ring (the C9-C10 bond), and (2) a unique oxidative rearrangement that allows for stereospecific migration of an alkyl group from a C9 quaternary center to C10. Overall, a pathway from enantiodefined epichlorohydrin to either enantiomer and/or diastereomer of a tetracyclic terpenoid skeleton was achieved by way of a highly modular route that proceeds in just a handful of chemical steps. We believe that the high step-economy and modularity are particularly powerful characteristics of the science established, and look forward to witnessing the impact of this contribution on future efforts in natural product and function-oriented synthesis campaigns.
In addition to presenting the organic chemistry central to our advance, we include an application of the science to the asymmetric synthesis of a classic tetracyclic terpenoid motif – the core skeleton of the natural product euphol (an agent related in structure to lanosterol that has a long history of use in traditional medicine that is known to possess anti-viral, anti-inflammatory and TGF-beta modulating properties). We prepared a model system possessing all quaternary centers about the tetracyclic skeleton and housing a cyclopropane at C14-C15.
Discovery of a Potent and Selective Agonist of the Estrogen Receptor Beta (ERb): Our efforts in organic chemistry have resulted in the discovery of a uniquely potent and selective natural product-inspired agonist of the estrogen receptor beta (ERb). It was recognized that one of the cucurbitane-like synthetic intermediates produced with our chemistry has molecular features that we considered may define it as a structurally unique and selective modulator of estrogen receptors. Initial assessment by Indigo Biosciences confirmed this expectation, revealing that we had prepared an exceptionally potent and selective agonist of ERb – a nuclear hormone receptor of great current pharmaceutical interest for a wide variety of indications including neurodegeneration and cancer. Collaboration with Professor Thomas Burris, an expert in nuclear hormone receptor biology at St. Louis College of Pharmacy and Washington University School of Medicine in St. Louis, then determined that our novel tetracyclic terpenoid-inspired agents agonize ERb in a prostate cancer cell line (DU-145).
Discovery of a Small Molecule as a Lead for the Treatment of Glioblastoma: Blessed with an outstanding collaborative partner in the Department of Neurology at the Geisel School of Medicine and the Norris Cotton Cancer Center, Professor Arti Gaur, we moved forward as a growing scientific team to explore the potential medicinal value of molecules now accessible from our chemical advance. To our great delight, Professor Gaur and her team discovered that our ERb-targeting agents possessed potent and selective toxicity toward glioblastoma (in comparison human neural stem cells and human astrocytes).
While we are moving forward with the goal of translating this discovery in basic biomedical science to a therapeutic for the treatment of glioblastoma, as well as a variety of other indications where selective agonism of this nuclear hormone receptor is thought to be potential clinical value, we are still in the early phases of developing what we hope will emerge as a powerful technology platform capable of driving the discovery of scores of novel natural product-inspired compositions of matter with unique medicinally-relevant properties.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in