After the Paper | Photophysics in Thiazolo[5,4-d]thiazole Phenol (TzTz-ph) Crystalline Aggregates
Published in Chemistry
![After the Paper | Photophysics in Thiazolo[5,4-d]thiazole Phenol (TzTz-ph) Crystalline Aggregates](https://images.zapnito.com/cdn-cgi/image/metadata=copyright,fit=scale-down,format=auto,quality=95/https://images.zapnito.com/users/206184/posters/1604986383-43-3595/bcf06949-38d8-4454-9956-67f52e1359be_large.jpeg)
Last year, our group published the article “Mechanochromism induced through the interplay between excimer reaction and excited-state intramolecular proton transfer” in Communications Chemistry. This work clarified the mechanism of excited-state intramolecular proton transfer (ESIPT) coupled excimer formation in the molecular crystal of CF3-HTTH (2,2′-(thiazolo[5,4-d]thiazole-2,5-diyl)bis(4-(trifluoromethyl)phenol). Such excited-state processes render mechanochromism, which was verified by the two-photon-excitation fluorescence images under different depths of crystals. We found that the molecules CF3-HTTH on the crystal surfaces exhibit monomeric emission; In contrast, the significant excimeric emission can be observed in the crystal kernel. The results indicated that the amorphous packing arrangements are dominant on the crystal surface, thereby prohibiting the formation of excimers.
Though all the experimental results seem to fit our interpretation, several puzzles are still unsolved: How could ESIPT couples excimer formation? What are the criteria for simultaneously undergoing ESIPT and excimer formation in the molecular solids? From the other perspective, could ESIPT processes become “collective behaviors” in molecular aggregates (Figure 1)? We believe that these interesting issues can be verified in the TzTz-ph molecular series due to the equilibrium-type ESIPT with high emission quantum yield, which is more accessible by the time-resolved emission spectroscopies.
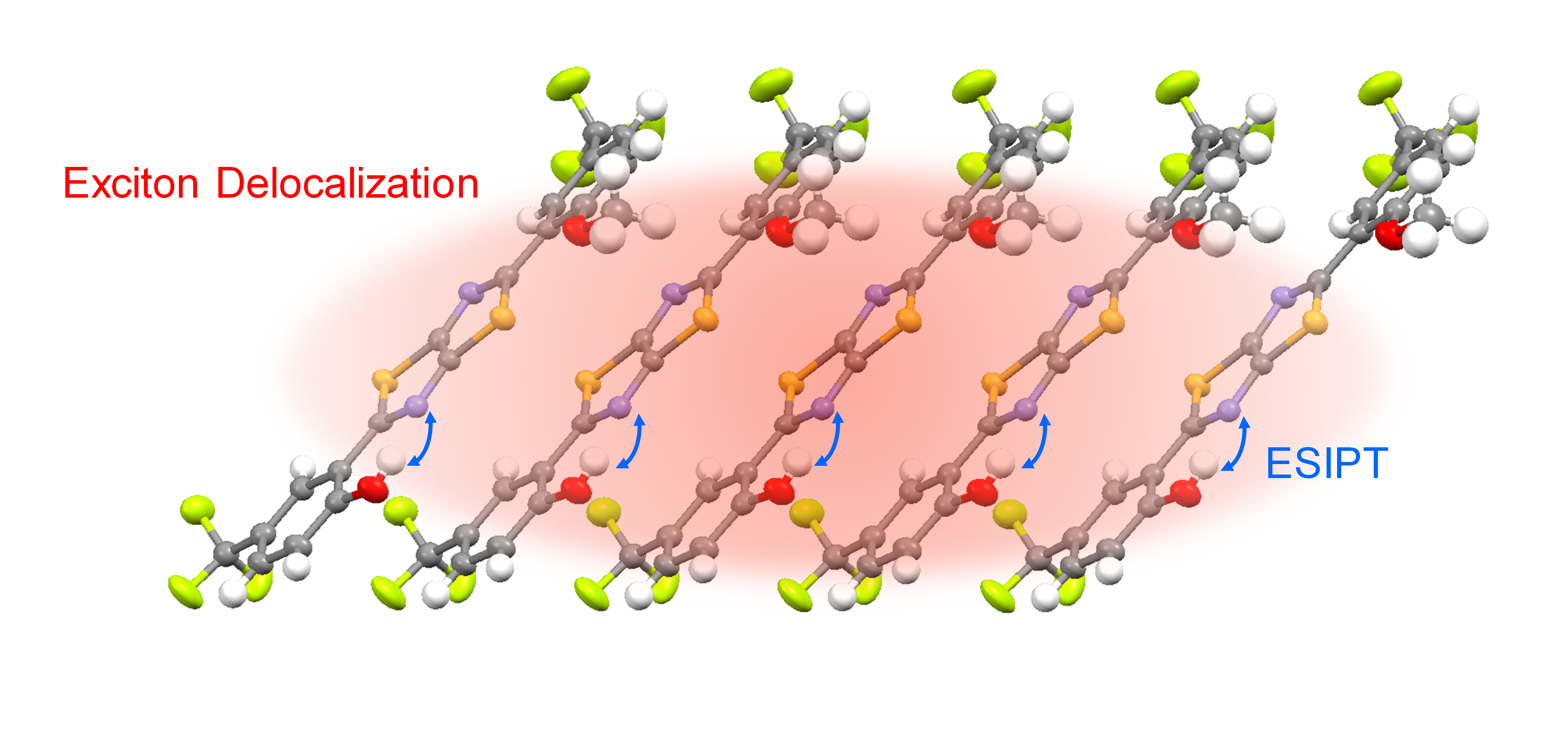
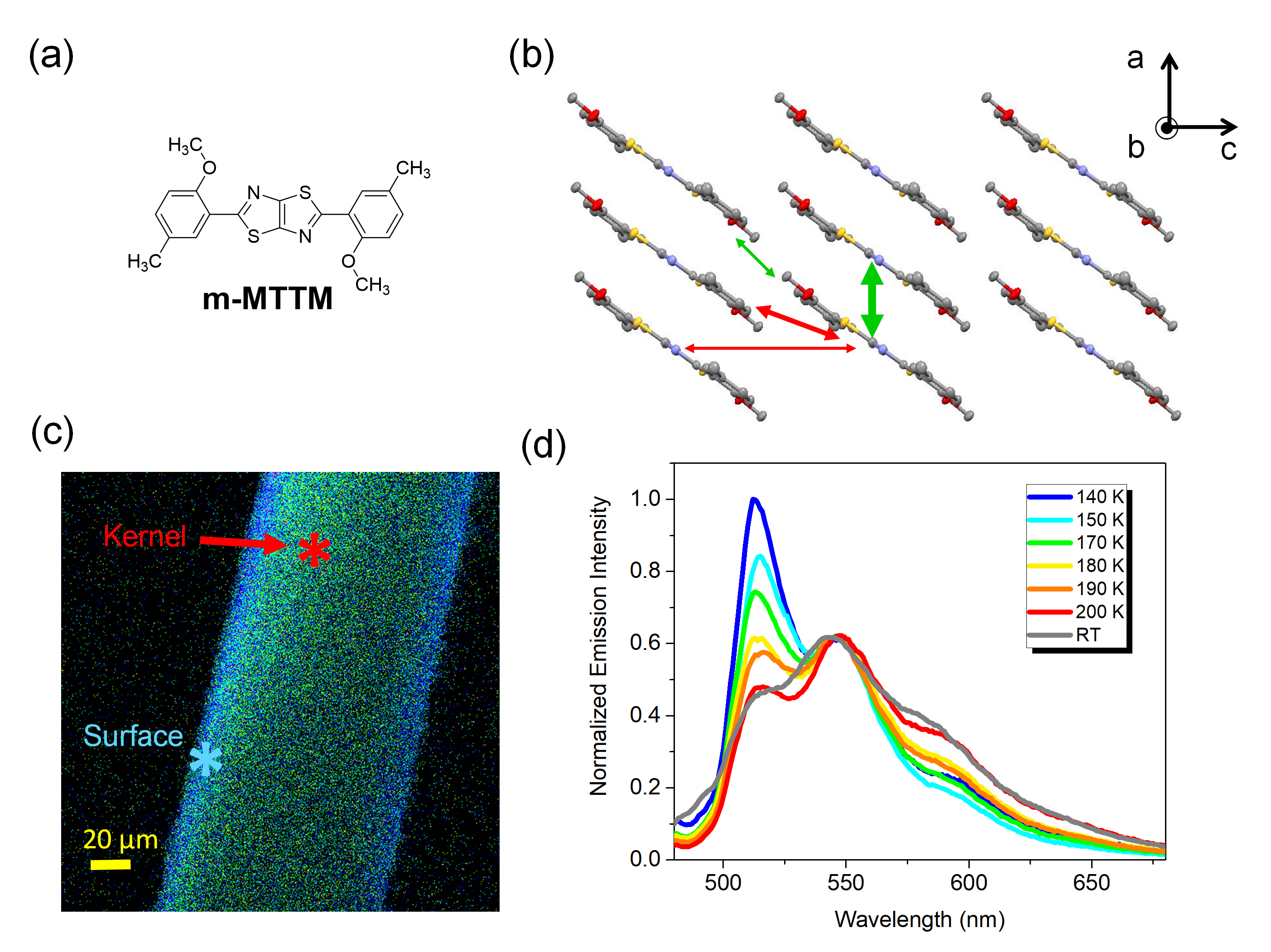
To simplify these issues, we start from the methylated TzTz-ph molecular crystals, which does not undergo ESIPT, to study the properties of exciton delocalization. We find that the molecular crystals of 2,2'-(thiazolo[5,4-d]thiazole-2,5-diyl)bis(4-methylphenol) m-MTTM (Figure 2a), which can be viewed as segregated HJ aggregates with opposite coupling strength along with different directions (Figure 2b), show the significant redshifted optical transition in the crystal kernel following the surface-kernel effect (Figure 2c). In addition, the temperature-dependent emission spectra show the rise of the 0-0/0-1 emission intensity ratio, which is the character of J aggregates (Figure 2d).
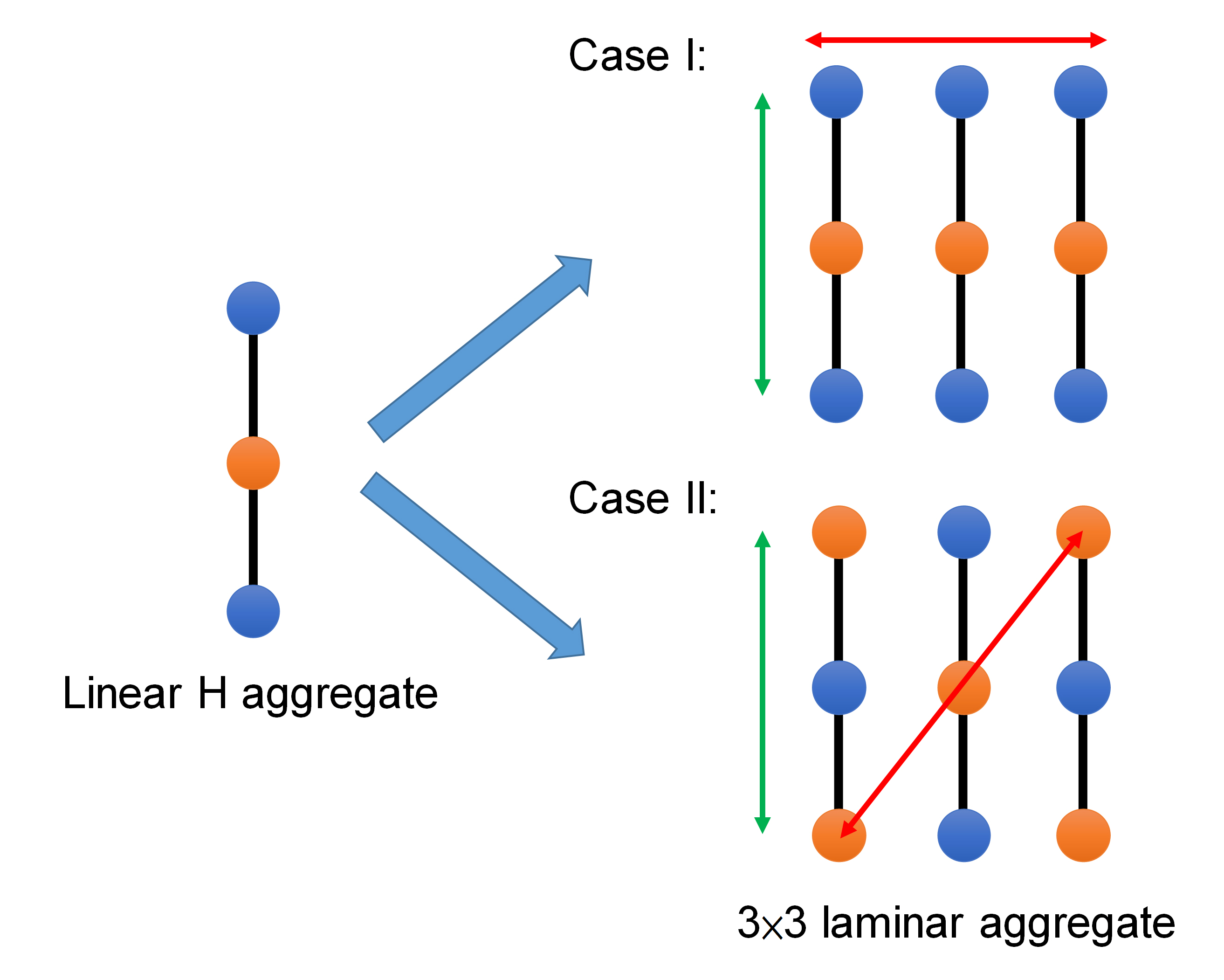
To clarify these phenomena, we calculate the exciton coupling along with different directions in the crystals and apply the Holstein model to simulate the absorption/emission spectra, validating that the J aggregates can form in the multiple linear H aggregates in the interchain direction (Figure 3), rendering the J-aggregate photophysical behaviors. The results together with the intriguing details are published in The Journal of Physical Chemistry A (Through-Space Exciton Delocalization in Segregated HJ-Crystalline Molecular Aggregates).
During this work, Dr. Shin-Wei Shen measured the photoelectron spectra and the X-ray diffractograms. Dr. Cheng-Ham Wu conducted the measurements of two-photon excitation fluorescence images. Prof. Zhiyun Zhang designed and synthesized the molecule m-MTTM. Prof. Yuan-Chung Cheng, Dr. Chern Chuang and Prof. Liang-Yan Hsu provided key suggestions to set up the theoretical models. I’d like to thank all of them for their contribution. Now we know the extend of exciton delocalization in the TzTz-ph molecular aggregates. Based on the results and analytical tools, we expect that the puzzles of ESIPT coupled excimer formation and the mechanism of “collective ESIPT” in molecular aggregates can be elucidated in the future.
Follow the Topic
-
Communications Chemistry

An open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of the chemical sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Experimental and computational methodology in structural biology
Publishing Model: Open Access
Deadline: Apr 30, 2026
Advances in Asymmetric Catalysis for Organic Chemistry
Publishing Model: Open Access
Deadline: Mar 31, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in