Breaking the Kinetic Barrier: A Decoupling–Recoupling Strategy for Converting Polyethylene to Ethylene and Propylene
Published in Chemistry
Achieving a circular economy depends on making smarter use of carbon resources. One of the biggest hurdles is how to turn waste plastics into something valuable again. Mechanical recycling can extend a plastic’s life but often reduces its quality, a process known as down-cycling. Chemical recycling, on the other hand, aims higher. By breaking polymers back down into their original monomers, it offers the possibility of closed-loop recycling. This approach is especially important for polyolefins such as polyethylene (PE) and polypropylene (PP), which make up a large portion of global plastic waste. Yet their strong carbon–carbon bonds make it difficult to selectively convert them into useful monomers like ethylene and propylene.
The Core Challenge: Kinetic Entanglement
In our earlier work published in Nature Chemistry (2024, 16, 871-880), we reported a layered self-pillared zeolite (LSP-Z100) featuring strong and accessible acid sites capable of interacting with bulky polymer molecules. This catalyst efficiently converted PE into gasoline-range hydrocarbons in a closed reactor. Building on this foundation, we aimed to convert PE to light olefins, specifically ethylene and propylene. Using a fixed-bed reactor at 260 °C, we tested PE conversion on LSP-Z100 and systematically refined the reaction conditions. The optimized process achieved a total olefin yield of 94% within two hours. However, most products were C4 and C5 olefins, while the yield of the targeted ethylene and propylene remained low at around 5%.
To further convert the intermediate products into ethylene and propylene, we introduced a second catalyst layer containing phosphorus-modified HZSM-5 (P-HZSM-5). The MFI-type channels and phosphorus modification, which partially reduces overly strong acid sites, may allow this zeolite to selectively crack the C4 and C5 olefins intermediates formed in the first stage into ethylene and propylene. This dual-layer catalytic system increased the yield of ethylene and propylene to 24%, while reducing the proportion of butenes and pentenes to 23%. However, the reaction also produced more by-products such as alkanes and aromatics.
We recognized the core challenge in this reaction system: the main and side reactions rely on the same acid sites and occur under identical conditions. This overlap creates a complex network of parallel and consecutive reactions, including cracking, oligomerization, hydrogen transfer, and aromatization, that take place simultaneously on acid catalysts. Such kinetic entanglement intrinsically links the formation of the desired light olefins (ethylene and propylene) with that of various by-products, including alkanes and aromatics. This fundamental constraint explains why conventional catalytic systems have long struggled to achieve high yield of ethylene and propylene.
Kinetic Decoupling-Recoupling Strategy
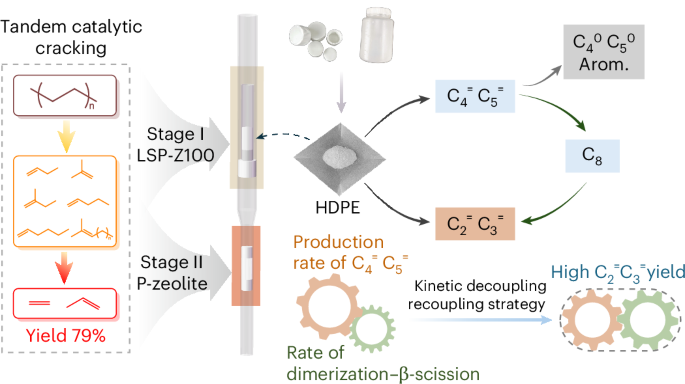
To overcome the challenge of kinetic entanglement, we developed a Kinetic Decoupling-Recoupling (KDRC) strategy using a two-stage reactor. This approach separates the reaction into two distinct steps. In the first stage, the LSP-Z100 catalyst selectively cracks PE into hydrocarbon intermediates, mainly C4 and C5 olefins, under mild conditions. In the second stage, the P-HZSM-5 catalyst converts these intermediates into ethylene and propylene through a dimerization-β-scission at high temperatures. Kinetic analysis revealed that the dimerization-β-scission (main reactions) is first-order reaction, while the hydrogen transfer and aromatization (side reactions) is second-order reaction. Maintaining an optimal intermediate concentration (KSS I) in Stage I by controlling the reaction temperature between 250 °C and 300 °C maximizes the rate difference between the main and side reactions. In Stage II, the conversion of C4 olefins to ethylene and propylene requires temperatures above 500 °C (KSS II, k4 > k3). Our two-stage KDRC system enables independent control over the kinetics of each stage, thus effectively suppressing by-product formation and raising the combined yield of ethylene and propylene to 79%.
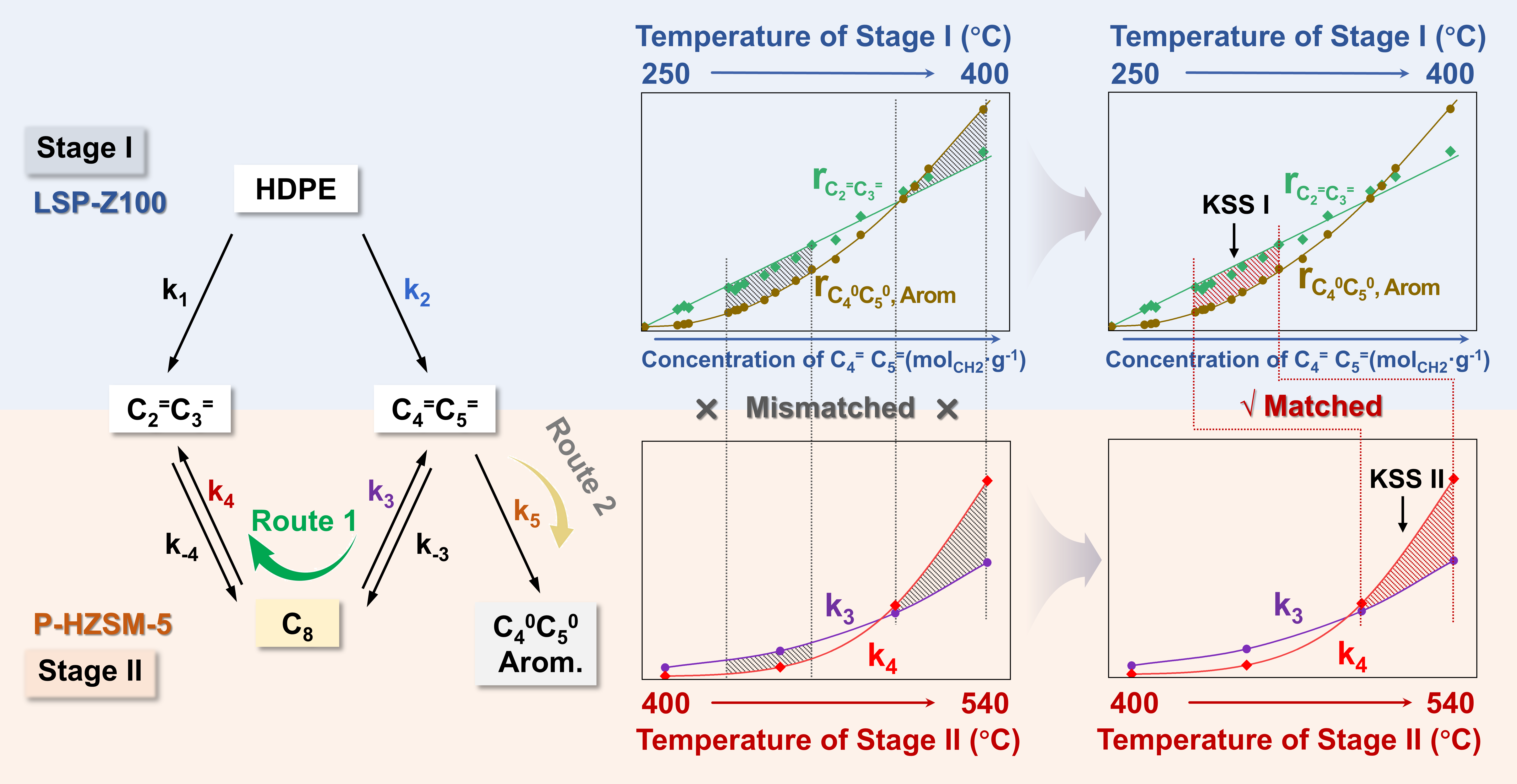
Fig. 1 Simplified reaction scheme proposed for PE conversion and optimized results over KDRC strategy.
Using synchrotron-based vacuum ultraviolet photoionization mass spectrometry (SVUV-PIMS), we directly detected C8 intermediates, confirming the dimerization–β-scission pathway in butene conversion. In situ neutron powder diffraction (NPD) further identified the locations of acid sites and adsorbed species, showing that phosphorus modification reduces acid site density, thereby limiting bimolecular reactions and enhancing selectivity toward ethylene and propylene.
Conclusion and Future work.
In conclusion, the KDRC strategy exhibits outstanding efficiency in selectively converting PE into ethylene and propylene, achieving high yields without the need for noble metals or external hydrogen. The durability of the catalysts and the system’s tolerance to common plastic additives highlight its strong potential for practical application. Looking ahead, efforts will focus on scaling up the reaction system and optimizing continuous-flow operations to enable industrial deployment. Developing efficient pretreatment methods to address deactivating additives, particularly basic nitrogen-containing compounds, will also be key to processing mixed plastic waste under real-world conditions.
For detailed experimental procedures, characterization data, and full kinetic analysis, please refer to our comprehensive article published in Nature Chemical Engineering: https://www.nature.com/articles/s44286-025-00290-y.
Follow the Topic
-
Nature Chemical Engineering

This is a new monthly online journal dedicated to publishing the most significant original research, commentary and analysis of direct relevance to the diverse community of chemical engineers.


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in