Capturing living tumor cells using soft DNA hydrogel
Published in Protocols & Methods

Conventionally, CTCs were isolated by microfluidic methods or nanostructured solid interfaces1-2. The cell damage or fragmentation is unavoidable because of the direct collisions between the CTCs and the hard microcolumn or the solid interface in the capturing process. Besides, the conventional release approaches such as enzymatic treatment and electrochemical repulsion failed to release the captured CTCs without cell damage. Accordingly, we attempt to create an approach using flexible, soft material to capture CTCs and explore a new way to release CTCs with minimal cell damage.
We chose to use DNA molecule, a biocompatible and nontoxic biomolecule to construct a flexible and soft material. Using a small piece of specific DNA as an initiator, the 3D porous DNA hydrogel can be assembled based on a clamped hybridization chain reaction. Using gold nanoparticles (AuNPs) as an indicator, we can visualize the formation of the DNA hydrogel. Figure 1 shows the soft DNA hydrogel with the pattern of “SJTU” (Shanghai Jiao Tong University).
Figure 1. Pattern of “SJTU” assembled by DNA hydrogel.
Aptamer that can specifically bind to the epithelial cell adhesion molecule (EpCAM) on the surface of CTCs is pre-hybridized with the initiator to trigger the clamped hybridization chain reaction. Due to the aptamer-initiator complex binding to EpCAM on the surface of CTCs, the 3D DNA hydrogel can be formed on the surface of CTCs by encapsulating the CTCs with a layer of soft DNA hydrogel (Figure 2). The soft property of the 3D DNA networks on cell surface can minimize the cell damage by encapsulating CTCs with high cell viability.
To achieve efficient CTC release with minimal cell damage, we introduce a small molecular responsive aptamer in the clamped hybridization chain (ATP aptamer was used in this work). When ATP is introduced, the 3D network that constructed by double-stranded DNA will be destroyed due to the ATP-binding induced DNA network dis-assembly (Figure 2). Given that ATP is one of the ubiquitous small molecules in biological system, it is biocompatible enough to be as the DNA hydrogel dis-assembly inducer for CTC release with minimal cell damage.
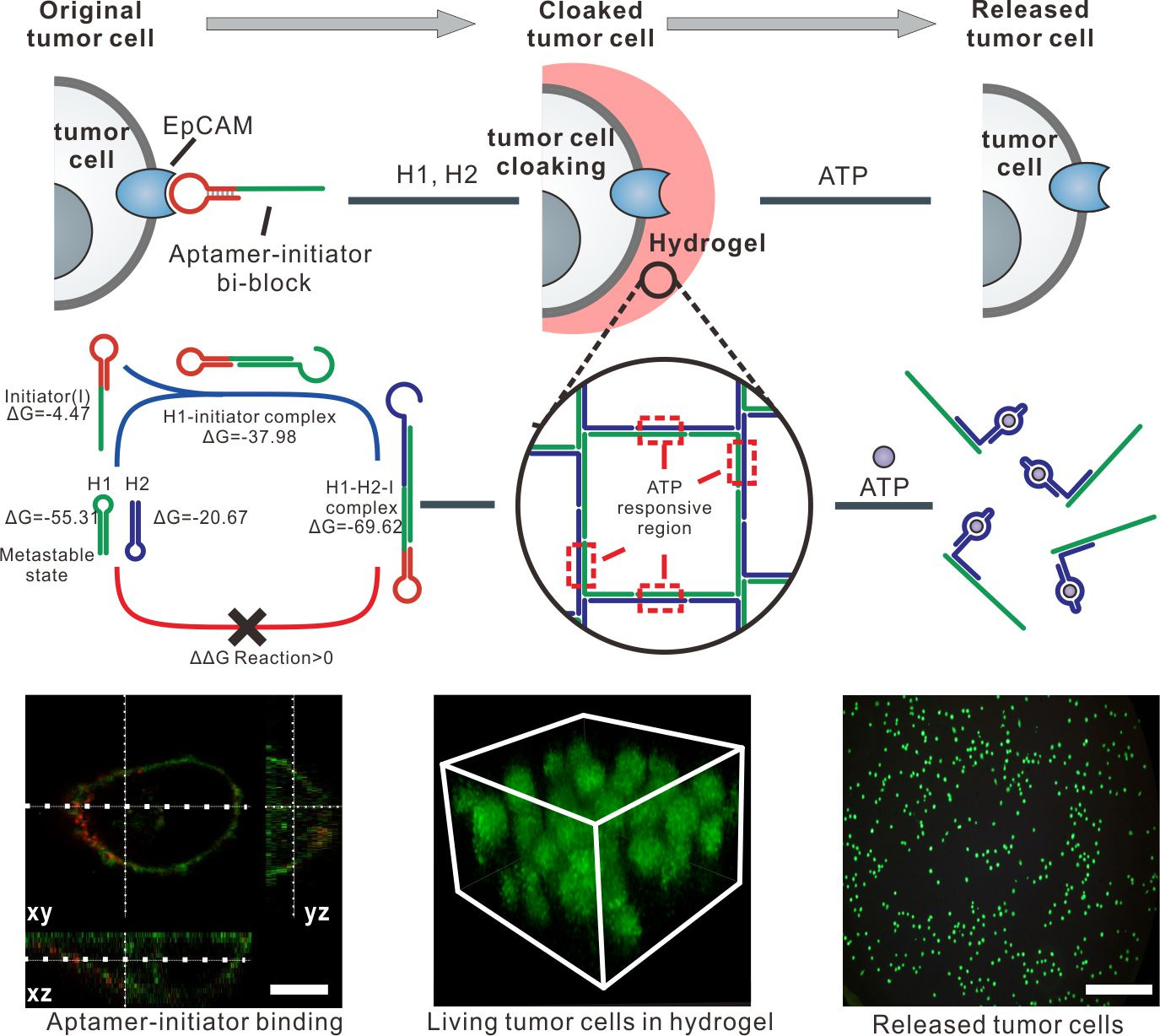
Figure 2. Encapsulation and release of living tumor cells.
In summary, the 3D porous DNA hydrogel with high softness and high biocompatibility opens the opportunity to isolate and release CTCs with high cell viability by providing a gentle capturing and releasing environment. We expect this new liquid biopsy tool to open new powerful and effective routes for cancer diagnostics and therapeutics.
You can find the online publication from now on via: https://doi.org/10.1038/s41596-020-0326-4
References
1. Liu, X.; Wang, S., Three-dimensional nano-biointerface as a new platform for guiding cell fate. Chem. Soc. Rev. 2014, 43 (8), 2385-2401.
2. Liu, L.; Sun, B.; Pedersen, J. N.; Aw Yong, K.-M.; Getzenberg, R. H.; Stone, H. A.; Austin, R. H., Probing the invasiveness of prostate cancer cells in a 3D microfabricated landscape. Proc. Natl. Acad. Sci. U. S. A.2011, 108 (17), 6853-6856.
Follow the Topic
-
Nature Protocols

This journal publishes secondary research articles and covers new techniques and technologies, as well as established methods, used in all fields of the biological, chemical and clinical sciences.
Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in