Computational pathology identifies immune-mediated collagen disruption to predict clinical outcomes in gynecologic malignancies
Published in Cancer, Computational Sciences, and Biomedical Research
Summary:
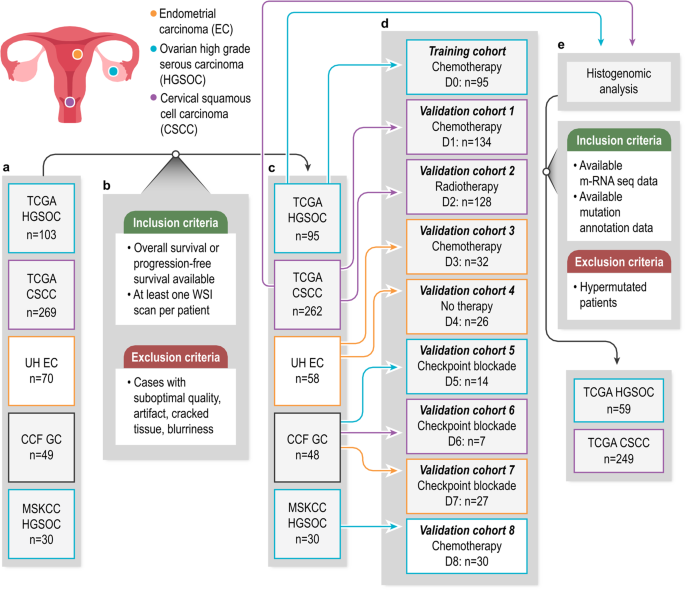
The study delves into the unclear role of immune cells in altering collagen structure within the tumor microenvironment (TME) of gynecologic cancers such as ovarian, cervical, and uterine cancers. A new computational method, CollaTIL, was developed to investigate how immune cells impact collagen architecture in these tumors and their consequent influence on cancer progression.
The findings revealed that heightened immune cell presence correlated with disorganized collagen structures within the tumor, while lower immune cell presence resulted in more organized collagen. Moreover, CollaTIL identified specific patterns associated with patient outcomes in these cancers. For instance, in cervical squamous cell carcinoma (CSCC), CollaTIL-associated features linked with gene signatures related to TCA-Cycle, and in high-grade serous ovarian cancer (HGSOC), they correlated with amino acid metabolism and macrophages.
This study's insights into the relationship between immune cell infiltration and collagen structure offer potential for significant advancements in gynecologic oncology. Integrating CollaTIL with genomic analysis presents promising prospects for devising improved therapeutic strategies and better prognostic assessments in the management of gynecologic cancers. Ultimately, these findings contribute to a deeper understanding of tumor biology and may aid clinicians in predicting disease progression risks for their patients.
Inspiration
The intricacies of the tumor microenvironment (TME) have unveiled a profound interplay between various cellular components and signaling molecules, dictating the trajectory of cancer progression and treatment response. At the epicenter of this complex network lies collagen, a fundamental protein in the extracellular matrix (ECM), exerting substantial influence over cancer metastasis and advancement.
Moreover, the dynamic participation of immune cells, particularly tumor-infiltrating lymphocytes (TILs), in shaping the ECM through enzymatic activity has come to the forefront of recent investigations. The intriguing correlation between the immune response and the breakdown of collagen architecture within the TME has sparked pivotal inquiries regarding their interconnectedness.
While machine learning strategies have proven adept at assessing the immune milieu in Whole Slide Images (WSIs), a substantial research gap persists in quantitatively evaluating the impact of immune modulation on collagen architecture within the TME. Our endeavor to bridge this gap led to the development of CollaTIL, a sophisticated computational pathology tool.
CollaTIL aims to unravel the intricate relationship between immune and collagen components within the TME of gynecologic cancers, particularly high-grade serous ovarian carcinoma (HGSOC), cervical squamous cell carcinoma (CSCC), and endometrial carcinoma (EC). Through quantitative characterization, we seek to discern how immune infiltration influences collagen architecture and, consequently, impacts tumor behavior and patient outcomes.
Our study not only sheds light on the quantitative characterization of the immune-collagen relationship but also ventures into molecular exploration by integrating genomic analysis. The CollaTIL-derived features reveal compelling associations between immune infiltrate, collagen architecture, and specific gene signatures, uncovering underlying biological pathways and potential biomarkers for disease prognosis.
Ultimately, our findings unveil a compelling correlation: heightened immune activity within the TME corresponds to disrupted collagen architecture, indicating a potential avenue for therapeutic intervention. Moreover, the linkage between CollaTIL-derived features and distinct molecular pathways underlines the promise of personalized treatment strategies for gynecologic cancers.
Through CollaTIL, we embark on a journey to decipher the intricate dialogue between immune cells and collagen architecture, offering a deeper understanding of the TME's role in cancer progression and opening doors to novel therapeutic approaches.
Limitations and Future directions:
We acknowledge that our study had certain limitations. One notable limitation was the absence of specialized staining techniques, such as immunohistochemistry (IHC) or Verhoeff-Van Gieson (EVG) staining, which would have enabled a quantitative analysis of collagen fiber segmentation. This limitation stemmed from the unavailability of IHC and EVG-stained whole slide images for the cohorts used in our study. Moreover, the interplay between immune and collagen components within the TME can be bi-directional. Additionally, we did not consider different types of immune cells in the TME, such as tumor-associated macrophages, which also play a critical role in ECM remodeling. Future work will focus on exploring the roles of different types of immune cells in the TME and their impact on collagen architecture.
Follow the Topic
-
Communications Medicine

A selective open access journal from Nature Portfolio publishing high-quality research, reviews and commentary across all clinical, translational, and public health research fields.
Related Collections
With Collections, you can get published faster and increase your visibility.
Reproductive Health
Publishing Model: Hybrid
Deadline: Mar 30, 2026
Healthy Aging
Publishing Model: Open Access
Deadline: Jun 01, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in