Controlling enzyme hydrolysis of branched polymers synthesised using transfer-dominated branching radical telomerisation via telogen and taxogen selection
Published in Materials
Worldwide polyester production has grown at a rapid rate in recent history. Global production of the commonly used polyester, polyethylene terephthalate is expected to reach around 35 million tonnes by 2030. Traditionally, industrial production routes to polyesters such as PET rely on step growth polymerisation reactions, which often suffer from negative environmental consequences such as significant wastewater evolution and stringent stoichiometric requirements. Within the last decade, Professor Steve Rannard and his research group have developed a novel technology termed transfer-dominated branching radical telomerisation, or TBRT for short, which is a facile, highly scalable chemical pathway that yields high molecular weight polymeric structures with step growth motifs, but which are prepared using chain growth mechanisms. Since the premier publishing of the strategy, the Rannard group has pushed TBRT down multiple avenues in a continued effort to establish this new technology in the world of polymer science. This effort has resulted in 10 publications, 2 patents, presentations at prominent international conferences. Significant industrial interest has led to a team of 6 scientists forming Polymer Mimetics Ltd., a joint venture between Scott Bader and the University of Liverpool that is working to utilise TBRT to develop innovative solutions for their customer base. In this study published in Communications Chemistry (Nature), we drive TBRT into the constantly evolving world of sustainable polymer chemistry by demonstrating that we can readily synthesise hyperbranched polyesters with tuneable molecular weight distribution, chemical functionality and hydrolytic susceptibility, all from an industrially viable chain-growth polymerisation.
Global production of the commonly used polyester, polyethylene terephthalate is expected to reach around 35 million tonnes by 2030. Traditionally, industrial production routes to polyesters such as PET rely on step growth polymerisation reactions, which often suffer from negative environmental consequences such as significant wastewater evolution and stringent stoichiometric requirements. Within the last decade, Professor Steve Rannard and his research group have developed a novel technology termed transfer-dominated branching radical telomerisation, or TBRT for short, which is a facile, highly scalable chemical pathway that yields high molecular weight polymeric structures with step growth motifs, but which are prepared using chain growth mechanisms. Since the premier publishing of the strategy, the Rannard group has pushed TBRT down multiple avenues in a continued effort to establish this new technology in the world of polymer science. This effort has resulted in 10 publications, 2 patents, presentations at prominent international conferences. Significant industrial interest has led to a team of 6 scientists forming Polymer Mimetics Ltd., a joint venture between Scott Bader and the University of Liverpool that is working to utilise TBRT to develop innovative solutions for their customer base. In this study published in Communications Chemistry (Nature), we drive TBRT into the constantly evolving world of sustainable polymer chemistry by demonstrating that we can readily synthesise hyperbranched polyesters with tuneable molecular weight distribution, chemical functionality and hydrolytic susceptibility, all from an industrially viable chain-growth polymerisation.
As a 3rd year undergraduate summer placement student, Dr. Savannah Cassin demonstrated that modified telomerisation conditions could be utilised to pro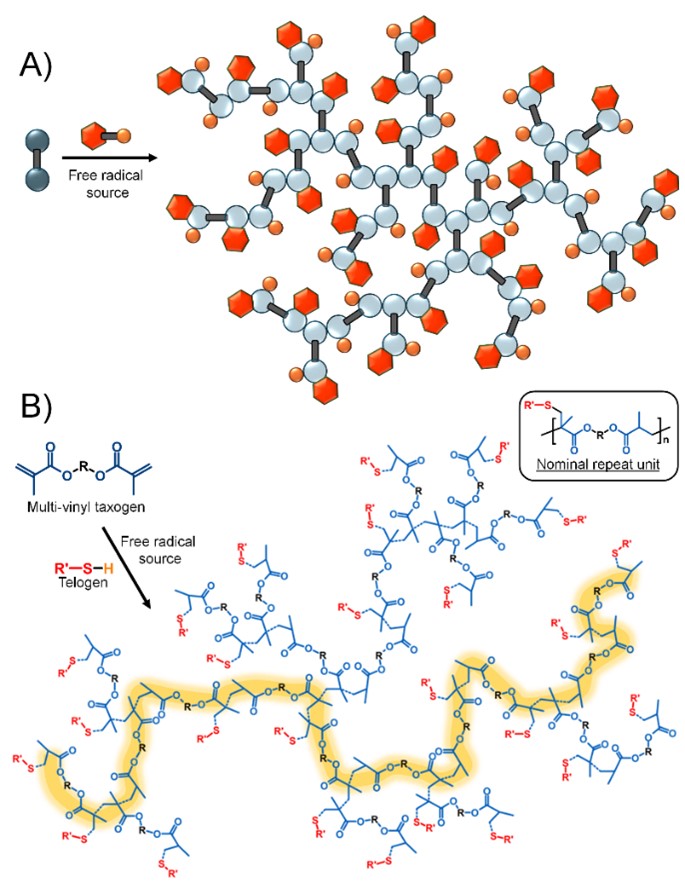 duce a homopolymer of the divinyl monomer, or taxogen, ethylene glycol dimethacrylate (EGDMA). This was the first time TBRT was conducted experimentally and from this research, multiple papers have been published exploring the mechanisms by which this reaction proceeds. Perhaps more interesting though are the structures of the macromolecules produced. These differ from most traditional chain-growth polymers as, instead of consisting of an inert carbon-carbon backbone, TBRT polymers synthesised from EGDMA, for example, contain polyester linkages within their extended macromolecular architecture. This generates potential for hydrolytic lability and subsequent use in biodegradable applications.
duce a homopolymer of the divinyl monomer, or taxogen, ethylene glycol dimethacrylate (EGDMA). This was the first time TBRT was conducted experimentally and from this research, multiple papers have been published exploring the mechanisms by which this reaction proceeds. Perhaps more interesting though are the structures of the macromolecules produced. These differ from most traditional chain-growth polymers as, instead of consisting of an inert carbon-carbon backbone, TBRT polymers synthesised from EGDMA, for example, contain polyester linkages within their extended macromolecular architecture. This generates potential for hydrolytic lability and subsequent use in biodegradable applications.
There is a growing school of thought amongst scientists that the landfilling of polymeric materials may be the most sustainable solution to polymer waste given the current explosion in plastic consumption the urgency to find immediate waste management solutions. This falls short in at least two cases. It is easy for scientists from developed countries, where correct infrastructure is in place, to champion landfilling techniques; however, polymer usage is a global challenge and there are many nations where local infrastructure may not sufficiently align with central governmental legislature. As a result, significant waste generation with little chance of remediation is likely to occur, particularly in developing nations. Additionally, formulation polymers in “down the drain” applications clearly cannot be landfilled, thus polluting the environment through waterways where sufficient treatment facilities are not present. To provide solutions for both scenarios, it is apparent that polymer biodegradability becomes a highly desired characteristic.
Biodegradability, especially in complex macromolecules such as polymers, is a hotly debated topic amongst the scientific community, not helped by the subject being politicised and droves of misinformation being constantly fed to the public. Governmental and regulatory body definitions are often a good starting point as they are typically developed with a level of scientific scrutiny. The Organisation for Economic Cooperation and Development (OECD) assesses the biodegradable potential of a material through standardised testing procedures such as in soil, fresh water and marine environments. Whilst these testing protocols are fairly rigorous, they can fall short in providing accurate assessments for extremely high molecular weight polymeric materials such as those synthesised by TBRT due to not accounting for oligomeric and molecular intermediates that may be produced as a result of early-stage polymer hydrolysis.
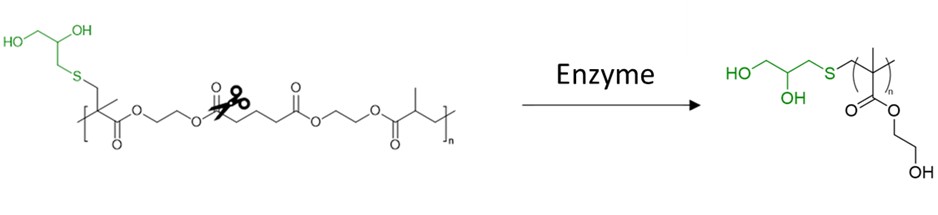
With careful consideration of the limitations of OECD testing, Dr. Samuel Mckeating, in  collaboration with Unilever, developed his own testing protocol that subjected TBRT polymers to different biotic hydrolytic stimuli. Preliminary data found that p(DDT-EGDMA) and p(DDT-LDMA), two polymers synthesised by TBRT, were not hydrolysed by enzymes in a suitable timescale to be considered as biodegradable candidates. To engineer hydrolytic susceptibility into TBRT polymers, A novel divinyl monomer, bisHEMA glutarate, was synthesised via base catalysed esterification of commercially available 2-hydroxyethyl methacrylate and glutaric acid. The resultant high molecular weight TBRT homopolymers synthesised from this monomer displayed significant vulnerability to enzyme catalysed hydrolysis, with a degradation profile comparable to polycaprolactone, a material classified as “readily biodegradable” by the OECD. We also discovered that altering the chain transfer agent, or telogen, chemistry of the TBRT polymer was a viable route to imparting degradative susceptibility into TBRT polymers. Considering that the TBRT polymers were highly branched with extremely high weight average molecular weight values, the tune-able hydrolysis was a significant result.
collaboration with Unilever, developed his own testing protocol that subjected TBRT polymers to different biotic hydrolytic stimuli. Preliminary data found that p(DDT-EGDMA) and p(DDT-LDMA), two polymers synthesised by TBRT, were not hydrolysed by enzymes in a suitable timescale to be considered as biodegradable candidates. To engineer hydrolytic susceptibility into TBRT polymers, A novel divinyl monomer, bisHEMA glutarate, was synthesised via base catalysed esterification of commercially available 2-hydroxyethyl methacrylate and glutaric acid. The resultant high molecular weight TBRT homopolymers synthesised from this monomer displayed significant vulnerability to enzyme catalysed hydrolysis, with a degradation profile comparable to polycaprolactone, a material classified as “readily biodegradable” by the OECD. We also discovered that altering the chain transfer agent, or telogen, chemistry of the TBRT polymer was a viable route to imparting degradative susceptibility into TBRT polymers. Considering that the TBRT polymers were highly branched with extremely high weight average molecular weight values, the tune-able hydrolysis was a significant result.
As previously mentioned, OECD testing protocols do not consider oligomeric and molecular intermediates when assessing polymer degradation. As a result, Sam used MALDI-TOF mass spectrometry to characterise the products of the degradation of TBRT polymers synthesised using the monomer bisHEMA glutarate. This analysis confirmed the hydrolysis of ester groups within the polymer backbone and confirmed the presence of small molecule and oligomeric degradation intermediates. The molecules characterised here can be considered analogous to some di- and tri-functional acids found in nature and thus indicate the potential of TBRT polymers for biodegradable applications.
With commercial relevance in mind, it was not enough to merely state that TBRT polymers undergo enzyme catalysed hydrolysis. Current and previous re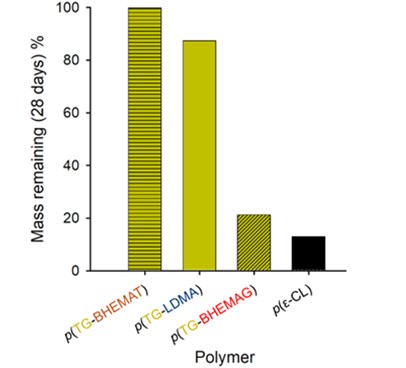 searchers within the Rannardresearch group, Dr. Sean Flynn, Dr. Savannah Cassin, Dr. Pierre Chambon, Dr. Oliver Penrhyn-Lowe and Stephen Wright have all demonstrated, in published research, that simple modifications to the polymerisation feedstock can result in dramatic structural and functional variation of TBRT polymers. Sam utilised this knowledge to modify both the taxogen and telogen chemistries within TBRT to alter the enzymatic susceptibility of resultant polymers, resulting in tuneable degradation over set time periods. This property of the polymers is of considerable interest in formulation and packaging applications, where delayed degradation will aid in preserving the shelf life of a material whilst improving its environmental impact. Sam also synthesised a bespoke taxogen, bisHEMA terephthalate, to resist enzyme catalysed hydrolysis and demonstrate further tuneability of TBRT polymer degradation.
searchers within the Rannardresearch group, Dr. Sean Flynn, Dr. Savannah Cassin, Dr. Pierre Chambon, Dr. Oliver Penrhyn-Lowe and Stephen Wright have all demonstrated, in published research, that simple modifications to the polymerisation feedstock can result in dramatic structural and functional variation of TBRT polymers. Sam utilised this knowledge to modify both the taxogen and telogen chemistries within TBRT to alter the enzymatic susceptibility of resultant polymers, resulting in tuneable degradation over set time periods. This property of the polymers is of considerable interest in formulation and packaging applications, where delayed degradation will aid in preserving the shelf life of a material whilst improving its environmental impact. Sam also synthesised a bespoke taxogen, bisHEMA terephthalate, to resist enzyme catalysed hydrolysis and demonstrate further tuneability of TBRT polymer degradation.
Following significant evidence of the tune-able biotic degradation of TBRT polymers, the Rannard group is looking to expand the technology further. Solvent free polymerisations, biosourcing, compostable materials and reversible curing are just some of the current avenues of research being undertaken by current PhD and Postdoctoral researchers. Where this research fits into the overall space of sustainable polymeric materials must be constantly assessed against relevant literature and regulation. With this in mind, we invite collaboration and shared discussion of ideas with both industrial and academic experts.
Follow the Topic
-
Communications Chemistry

An open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of the chemical sciences.
Ask the Editor – Polymers
Got a question for the editor about Functional polymers? Ask it here!
Continue reading announcementRelated Collections
With Collections, you can get published faster and increase your visibility.
f-block chemistry
Publishing Model: Open Access
Deadline: Feb 28, 2026
Experimental and computational methodology in structural biology
Publishing Model: Open Access
Deadline: Apr 30, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in