Diagnostic impact of whole exome sequencing in neurometabolic disorders in Syrian children: a single center experience
Published in Genetics & Genomics, General & Internal Medicine, and Paediatrics, Reproductive Medicine & Geriatrics
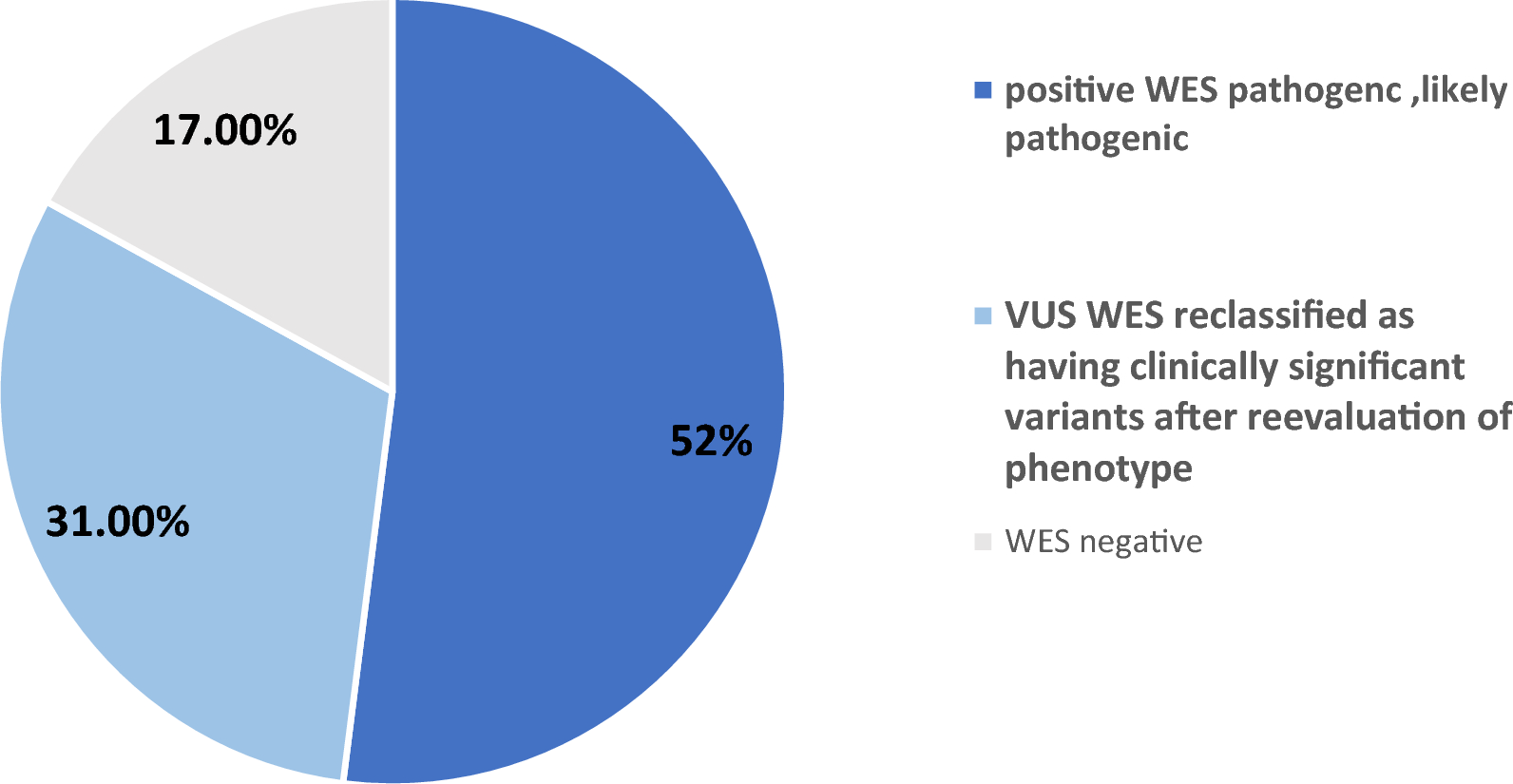
This study emerged from an urgent clinical need. We evaluated 54 children with unexplained neurological and metabolic symptoms at our center. Many of these patients had a history of consanguinity and had previously undergone long diagnostic journeys without clear answers. By applying WES, we identified pathogenic or likely pathogenic variants in 52% of cases and clinically relevant findings in 83%, including 14 novel mutations not previously reported.
Beyond diagnosis, WES had a direct impact on patient care:
Diagnostic reclassification occurred in 83% of cases
Treatment plans were adjusted in 74% of cases
Prognostic clarity and preventive counseling became possible for the majority of families
Our work highlights the transformative role of WES in diagnosing rare pediatric conditions, particularly in populations with high consanguinity and limited access to traditional genetic services. We hope this study encourages the integration of genomic diagnostics into neurology and pediatrics in similar settings worldwide.
We invite you to read our paper and join the conversation.
https://rdcu.be/ek5is
Follow the Topic
-
Orphanet Journal of Rare Diseases

An open access, peer-reviewed journal that encompasses all aspects of rare diseases and orphan drugs and publishes high-quality reviews on specific rare diseases.
Related Collections
With Collections, you can get published faster and increase your visibility.
Advances in Our Understanding of Glutamatergic Receptor Biology: Selected Papers from the 7th European GRIN Conference
This Collection showcases selected research presented at the 7th European GRIN Conference, where families, clinicians, and scientists gathered to explore the latest discoveries in GRI disorders.
The featured papers delve into the biology of NMDA and AMPA receptors, their gene variants, and their clinical implications, offering insights into complex symptomatology and emerging therapeutic approaches.
Reflecting the collaborative and translational nature of the event, this Collection bridges foundational science with real-world impact, aiming to improve outcomes for individuals affected by GRI-related conditions.
All submissions in this collection undergo the journal’s standard peer review process. Similarly, all manuscripts authored by a Guest Editor(s) will be handled by the Editor-in-Chief. As an open access publication, this journal levies an article processing fee (details here). We recognize that many key stakeholders may not have access to such resources and are committed to supporting participation in this issue wherever resources are a barrier. For more information about what support may be available, please visit OA funding and support, or email OAfundingpolicy@springernature.com or the Editor-in-Chief.
Publishing Model: Open Access
Deadline: Jun 15, 2026