From Models to Clinics: Translating Diabetic Retinopathy AI Research into Real-World Medical Imaging Systems
Published in General & Internal Medicine
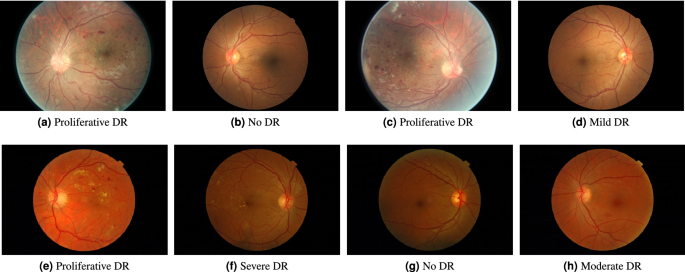
Diabetic retinopathy remains one of the leading causes of preventable vision loss, and medical imaging–based AI has become a powerful tool for early detection. Research in this area has demonstrated that deep learning models can match or exceed human performance under controlled conditions. Yet, deploying these models in real-world clinical workflows reveals challenges that are often invisible at the research stage.
One of the most immediate challenges is data variability. Retinal images collected across different clinics, camera devices, and acquisition protocols can differ significantly in quality, resolution, illumination, and noise. Models trained on standardized datasets frequently struggle when exposed to this diversity. Addressing this gap requires architectural robustness, careful preprocessing strategies, and validation across heterogeneous data sources rather than reliance on a single benchmark dataset.
Explainability is another critical requirement in medical imaging systems. Clinicians need to understand not only a model’s prediction, but also the visual reasoning behind it. Heatmaps, feature localization, and confidence scoring become essential components of the system rather than optional research artifacts. Without transparent decision support, even highly accurate models face resistance in clinical adoption.
Regulatory and compliance considerations further shape system design. In healthcare environments, model updates cannot be treated as routine software changes. Version control, traceability of training data, and reproducibility of results are essential for auditability. In practice, this means that AI models for diabetic retinopathy must be embedded within carefully governed pipelines that balance innovation with patient safety and regulatory accountability.
Perhaps the most valuable insights emerge after deployment. Real-world feedback often reveals edge cases such as rare retinal pathologies, imaging artifacts, or population-specific patterns that were underrepresented in the original training data. These observations frequently drive new research questions, closing the loop between clinical practice and academic investigation.
Translating diabetic retinopathy AI research into production medical imaging systems is not simply an engineering exercise. It is an extension of the research process itself, where models are continuously tested against reality. I believe that progress in this space will increasingly depend on researchers who can navigate both rigorous algorithmic development and the practical constraints of clinical deployment.
Follow the Topic
-
Scientific Reports

An open access journal publishing original research from across all areas of the natural sciences, psychology, medicine and engineering.
Related Collections
With Collections, you can get published faster and increase your visibility.
Obesity
Publishing Model: Hybrid
Deadline: Apr 24, 2026
Reproductive Health
Publishing Model: Hybrid
Deadline: Mar 30, 2026





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in