Graphene oxide in water: what we learn from accurate computer simulations.
Published in Chemistry
When I started my postdoctoral contract at the École Normale Supérieure (Paris, France), with ML Bocquet and F-X Coudert, we decided to study the chemical reactivity of graphene oxide (GO) in liquid water, with or without charged species (ions) in the liquid.
To reach that purpose, it was first necessary to generate a realistic GO layer. The difficulty started already because unlike the prototypical graphene layer, the GO layer is ill-defined at the atomic scale: how many oxidized functions present on the surface? Their nature? Their spatial arrangement over the graphene sheet?
We therefore generated different GO replicas, with the exact same chemical composition where epoxides and alcohol functions (see Figure below) are grafted randomly either on the entire graphene layer or on half of it. Using the Density Functional Theory (DFT) approach, we established that the GO replicas where the chemical functions tend to cluster are more stable, in agreement with recent experimental data (Raman spectroscopy, solid NMR).
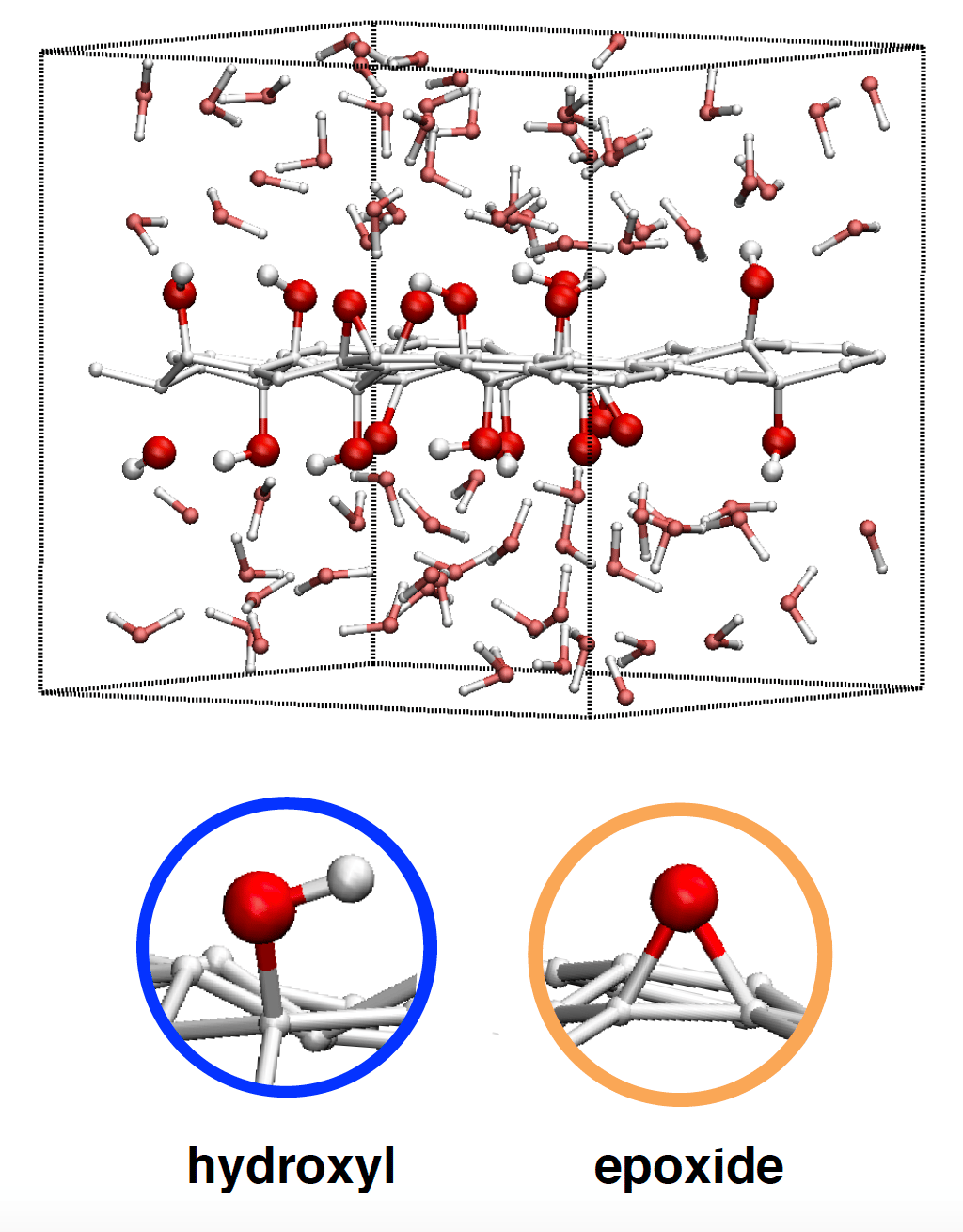
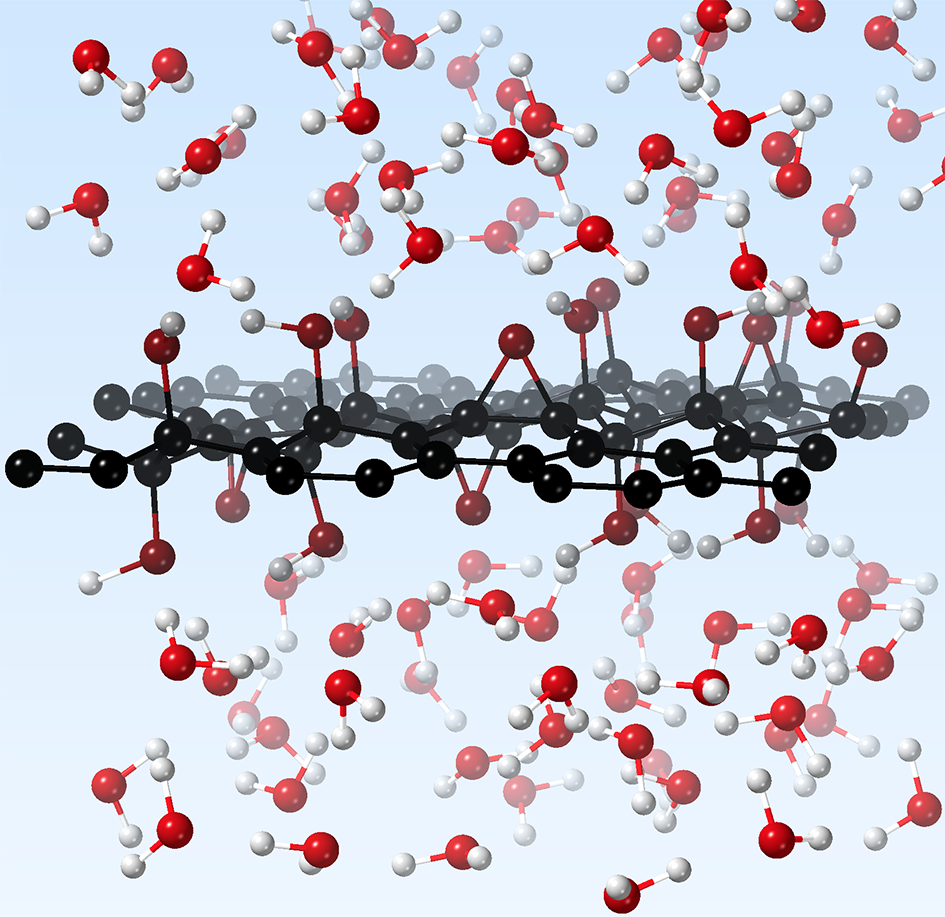
Figure 1: GO+water system in the simulation box. Periodic boundary conditions are applied but not represented here for the sake of clarity. Image performed with the Visual Molecular Dynamics software, version 1.9.3.
Then, we concentrated our efforts on the most stable GO replicas among the generated structures. We placed them into a simulation cell with liquid water solvating the GO layer at room temperature (T = 300 K).
To ensure the relevance of these preliminary ab initio Molecular Dynamics simulations, I decided to track the evolution of the GO+water system using a vizualisation software. I had the surprise to observe that the GO surface was unexpectedly reactive, even in the absence of charged species in the liquid.
We finally identified 3 types of reactive events:
- Opening/closing of the epoxide rings that can be converted into a zwitterion, with a positive and negative charge close to the carbon and oxygen atoms, respectively.
- Proton transfers between the surface -OH groups and the neighboring water molecules of the liquid. Such reactions create a net negative charge at the surface of the GO layer.
- A rarer and more spectacular dehydration reaction. Due to the spatial proximity of several hydroxyl groups, surface proton transfers between them are spontaneous and can form one adsorbed water molecule. The latter can then desorb and migrate to the bulk via an electronic rearrangement.
Identifying such reactive events would not have been possible without a state-of-the-art description of the electronic properties of this material. Therefore, from now on, we know that:
- the oxidized functions tend to cluster at the surface of the GO layer.
- GO should be modeled as a (partially) negatively charged surface when dipped into liquid water.
This work opens the way for more extensive ab initio studies of the GO and analogues in different conditions: influence of the confinement thickness on the water dynamics, charge effects induced by the presence of other ionic species...
The paper can be found here: https://www.nature.com/article...
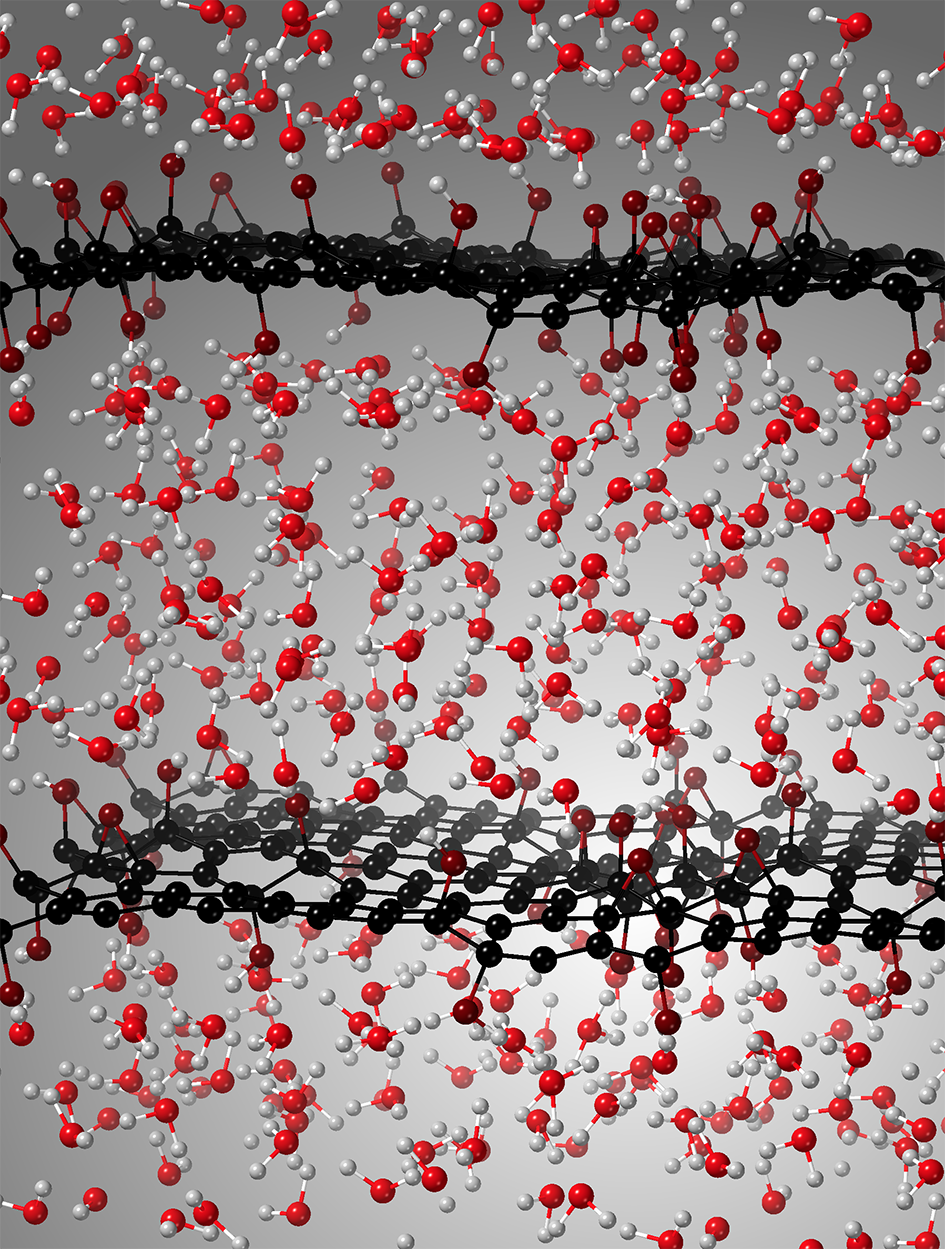
Figure 2: Periodic replicas in x,y,z directions of the GO+water supercell. Average distance between the carbon sheets is 1.4 nm. Carbon, surfacic oxygen, water oxygen and hydrogen atoms are represented by black, garnet red, red and light grey color balls respectively. Image performed using the graphic software Crystal Maker X, version 10.4.
The authors acknowledge funding from the Horizon 2020 FETOPEN project NANOPHLOW number 766972. This work was granted access to the HPC resources of TGCC and CINES under grants A5-A0070807364 and A0070807069 by GENCI. We also thank Vasu Kalangi and Lydéric Bocquet for insightful discussions about GO membranes.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in