How to boost mucosal IgA responses: learn from microbiome
Published in Microbiology, Immunology, and Public Health
Antibodies are one of the most important molecular weapons in defense against infections, but different types of antibodies have distinct functions. While IgG antibodies play a significant role in immune defense against infections and are the most extensively studied and utilized antibody type, their levels are much lower than IgA antibodies at the mucosal surfaces, such as the oral cavity, nasal cavity, and intestines. Therefore, IgA antibodies serve as the primary protective factor in certain mucosal infections. IgA antibodies can block the invasion of pathogens at the mucosal level, preventing or reducing their entry into the body’s internal organs. Thus, their protective effects sometimes exceed those of IgG antibodies. IgA also plays an important role in maintaining the stability of the intestinal microbiota. Patients with IgA deficiencies not only tend to exhibit recurrent mild respiratory, gastrointestinal, and other mucosal tissue infections, but also experience disruptions in the intestinal microbiota. Moreover, IgA also plays a crucial regulatory role in certain non-infectious diseases, such as autoimmune diseases and obesity. However, the development of IgA antibodies currently lags behind that of IgG antibodies. How to enhance the host’s IgA antibody response to mucosal infections remains unknown.
Vaccines are the most important and effective means of preventing and controlling infectious diseases. However, the strength of mucosal IgA responses generated by the same vaccine can vary greatly among different individuals, leading to varying levels of vaccine protection. In general, elderly people generally exhibit weaker responses to vaccines, resulting in relatively lower vaccine protection. Various factors such as gender, genetics, and chronic diseases can also influence the host’s response to vaccines. Currently, our understanding of the factors and regulatory mechanisms that affect the host’s production of mucosal IgA antibodies in response to external antigens is not comprehensive. When facing mucosal infections, the lack of necessary knowledge and means to artificially enhance the host’s mucosal IgA antibody response to external antigens is a significant challenge.
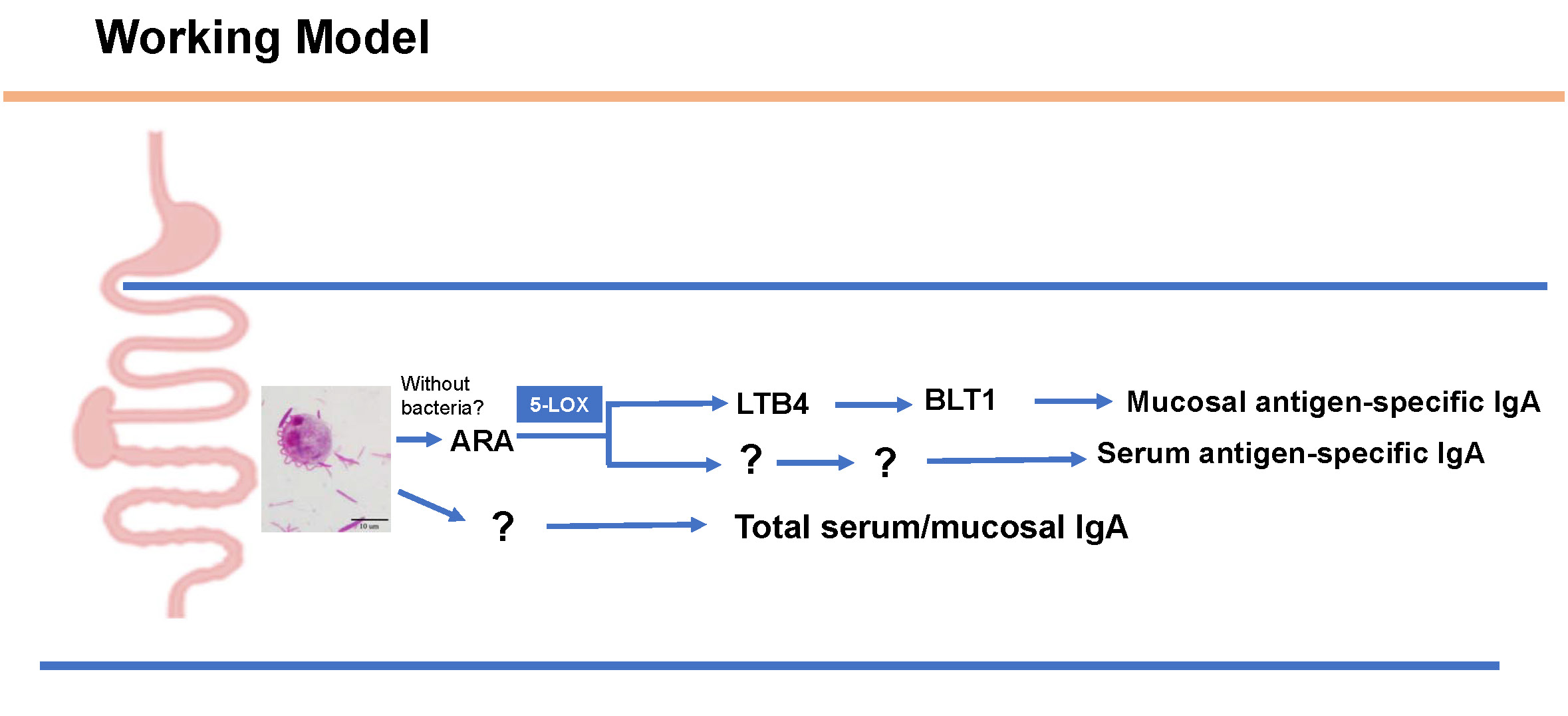
Our study focuses on factors influencing mucosal IgA responses, aiming to identify effective approaches and means to enhance the host’s mucosal protective function, and to promote the development of new strategies for vaccination that can effectively promote mucosal protection against infectious diseases. Our study reports an interesting observation that a mouse gut commensal protozoan boosting antigen-specific mucosal IgA responses. This enhancement appears to be related to the protist’s ability to modulate the intestinal microenvironment by altering the intestinal metabolome, particularly the levels of luminal arachidonic acid (ARA). Dietary supplementation of ARA or colonization with the protist were found to amplify antigen-specific mucosal IgA responses, contingent upon an optimal host metabolism and subsequent activation of a specific lipid-dependent signaling pathway. Further investigation on the role of diet and the microbiome on IgA responses have exciting potential to influence oral vaccinology and better understand mucosal immunity.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in
send me full paper Professor. Thank you my Email Id is b.deivasigamani@gmail.com
It is free available online:
https://www.nature.com/articles/s41467-024-52336-z
Thank you