Nanoparticle drug delivery targets an intracellular source of pain
Published in Bioengineering & Biotechnology
Sensing and responding to pain is an essential mechanism, and when exposed to a painful stimulus, our body initiates responses to avoid further damage and initiate appropriate wound healing processes. However, damaged tissue or chronic conditions (e.g. arthritis or diabetic neuropathy) can dysregulate pain pathways, leading to chronic pain, which is both debilitating and difficult to treat. While opioids remain the most effective treatment, there are many unwanted side-effects including tolerance (analgesic effect diminishes over time) and dependence, which are major contributors to the current, growing global opioid epidemic.
G protein-coupled receptors (GPCRs) are cell-surface sensors that play important roles responding to painful stimuli and relaying these pain signals from sensory neurons to the spinal cord and brain. Intriguingly, we and others have previously demonstrated that when GPCRs such as the Neurokinin 1 receptor (NK1R) are stimulated during pain, they undergo endocytosis and relocate to intracellular organelles such as the endosomal network.
Pharmaceutical companies have spent more than $1billion attempting to identify new non-opioid analgesics, with limited success - although potent, selective inhibitors have been identified, many have failed in clinical trials. We reasoned that a possibility for this lack of clinical success is that pain targets such NK1R remain “hidden” within neurons during chronic pain states, thus reducing drug accessibility to the target. Bypassing receptors on the cell surface and releasing the drug payload into endosomes may therefore direct the drug to an important source of pain and improve analgesia.
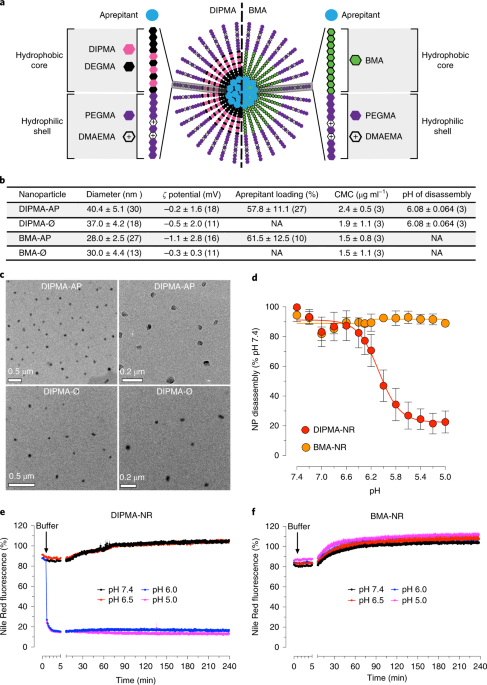
This study was only possible working as a multi-disciplinary international team, combining expertise in nanotechnology, neuroscience and GPCR pharmacology. We developed and tested pH-responsive, self-assembling soft polymeric nanoparticles that can be loaded with small molecule drugs for intracellular drug delivery when exposed to the acidic environment of endosomes. The drug of choice was the NK1R antagonist aprepitant, currently used in the clinic for emesis (and previously failed for the treatment of pain). To determine the efficacy of endosomal drug delivery for pain, we compared these nanoparticles to non-pH-responsive control particles or ‘free’ drug, and tracked fluorescently labeled particles in cells and animals. We then tested their efficacy in neurons, spinal cord slices and cell lines, followed by pre-clinical pain studies. Only pH-sensitive delivery improved aprepitant efficacy and provided sustained analgesia.
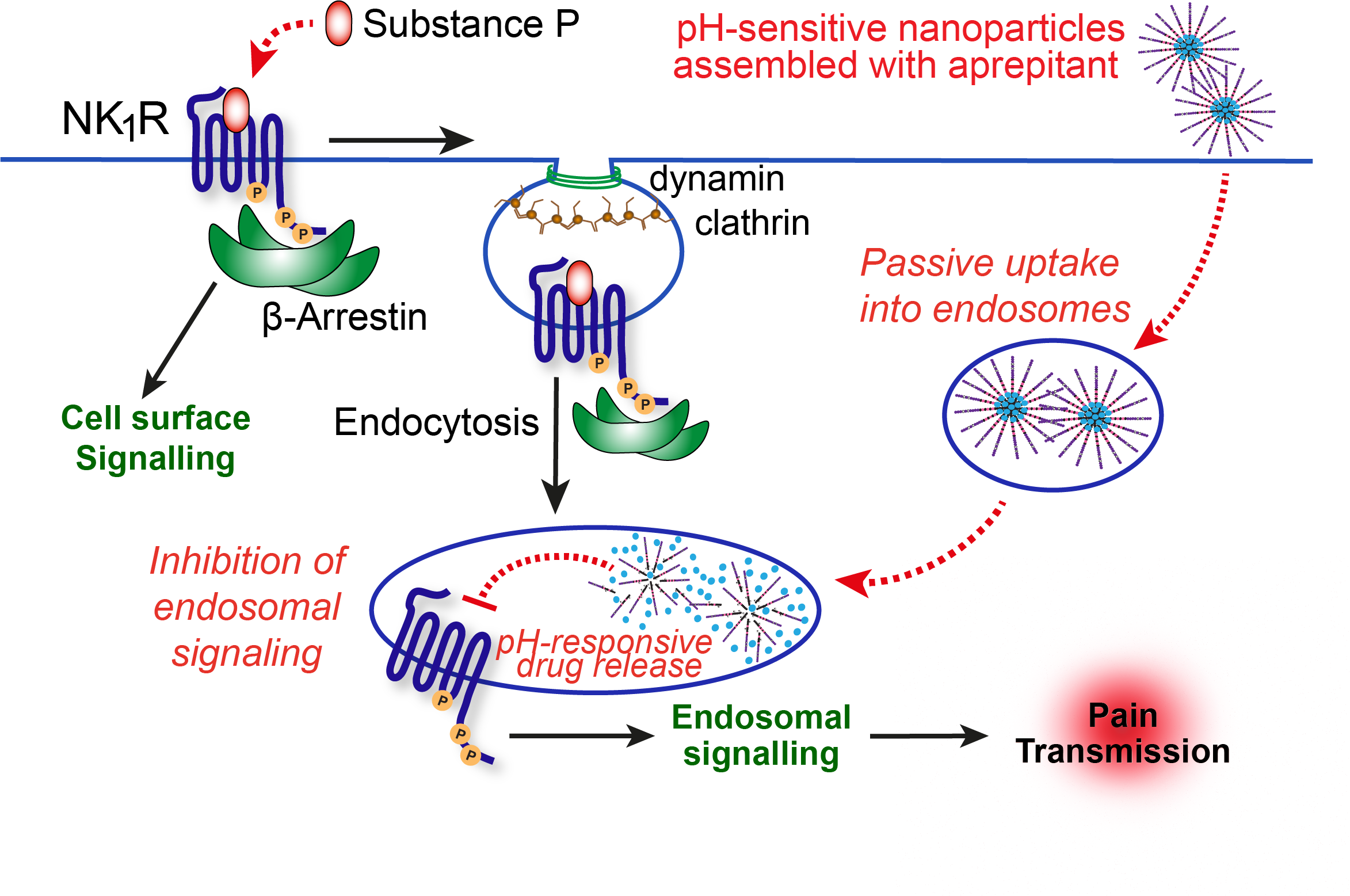
It is tempting to speculate that similar benefits could be achieved for other failed analgesic candidates. We are therefore very motivated to explore other drug candidates and stimulus-responsive materials in a similar manner, to provide further proof of concept that optimal formulation and drug targeting may improve analgesia and alleviate our reliance on opioids for treating pain.

Photo: PhD candidate Paulina Ramirez-Garcia and Dr Nicholas Veldhuis
Find out more about the study here: Paulina D. Ramírez-García et al., A pH-responsive nanoparticle targets the neurokinin 1 receptor in endosomes to prevent chronic pain. https://doi.org/10.1038/s41565-019-0568-x
Follow the Topic
-
Nature Nanotechnology

An interdisciplinary journal that publishes papers of the highest quality and significance in all areas of nanoscience and nanotechnology.





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in