New life of trifluorodiazoethane - new precursor and new generation condition
Published in Chemistry
Diazo compounds are a class of highly attractive synthons that are widely used in academic and industrial production. The search for room-temperature decomposable N-sulfonylhydrazones as precursors of unstable diazo compounds and exploration of their synthetic utilizations are one of the main goals of Bi group in the past years.
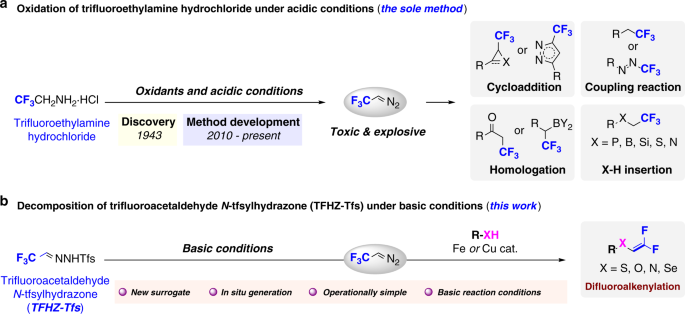
Trifluorodiazoethane (CF3CHN2) is a highly active fluoroalkylating agent that has commonly used in the construction of various trifluoromethyl group bearing structural motifs. At present, the main barrier to the development of this chemistry originates from the only generation strategy of CF3CHN2. The limited acidic oxidation conditions and the sole precursor (trifluoroethylamine hydrochloride) lead to poor chemical development. In 2018, we were thrilled to make a breakthrough in the generation strategy of CF3CHN2, by using TFHZ-Tfs as a CF3CHN2 surrogate, which is capable of generating CF3CHN2 in situ under basic conditions. In addition, the reagent has outstanding applicability in various transformations such as: difluoroalkenylation of X-H (X = N, O, S, Se), Doyle-Kirmse reaction and cyclopropanation. The discovery of this reagent not only provides a new alternative generation condition for CF3CHN2, but also constructs a number of novel fluorinated organic molecules. We believe that this new process will have more applications in the development of this chemistry or related field.
After the initial design and synthesis of TFHZ-Tfs, we first explored its reactivity with X-H nucleophile. We have found that TFHZ-Tfs could release CF3CHN2 in aqueous sodium hydroxide solution and reacts with thiophenol to give a difluoroalkenylated product. After searching the literature, we were so surprised to find that the traditional synthesis conditions for such structures are very harsh and have narrow substrate range. We have realized that the strategy of using TFHZ-Tfs to decompose under basic conditions may become a breakthrough in the construction of heteroatom-substituted gem-difluoroalkenes. Next, we conducted a continuous condition screening and tried a variety of X-H nucleophiles, including Se, N and O. Although the yields of different heteroatom products fluctuate, we were pleased to find that these substrates are feasible.
After obtaining these results, we wanted to verify whether it can be used as a qualified CF3CHN2 precursor by exploring the applicability of the reagent in other classical reactions, wherein we have chosen the most representative Doyle-Kirmse reaction and cyclopropanation reaction. The suitability of this reagent in both types of reactions is beyond our imagination, and it is very prominent in both reaction yield and stereoselectivity. Compared with the existing generation methods, the discovery and application of TFHZ-Tfs fills a gap in this chemistry and will provide a new platform for the construction of more fluorinated organic molecules.
For all of us, it is exciting to achieve such great success through hard work and good collaborations. Also, we would like to express our heartfelt gratitude to the referees of Nature Chemistry for their critical comments on our manuscripts. This enables both our authors and the synthetic methodology stronger and stronger and finally be suitable for Nature Communications.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in