
To understand the emergence of life, one needs to make compartments that can hold the biomolecules and chemical reactants – precursors of life – and that can synthesize peptides in a confined environment without any modern (bio)catalysts. The physiochemical properties of such compartments - protocells –could provide foundational insights into the nature of primitive cells and the chemical origin of life.
The formation of an amide bond is arguably the most important reaction in life, but also one of the most difficult reactions to catalyze, because of the usually strongly polarized amine and carboxylic acid groups. In modern biology, specialized aminoacyl-tRNA synthetases activate the carbonyl group, and its reaction with an amine is facilitated by positioning the aminoacyl-tRNAs in the ribosome active site. In peptide chemistry, carbonyl groups are also commonly activated before reaction with an amine. However, none of these routes were available on Early Earth, and an alternative route leading to effective peptide synthesis must have existed, given the relevance of peptides as building blocks and catalysts in all forms of life.
Emerging evidence has shown that aminothioacids could be plausible precursors to peptides, as they can be formed in aqueous solution from aminonitriles and be coupled together or to other amino acids by oxidative activation with ferricyanide1. However, so far, this reaction could not be localized in a small cell-like compartment and a selective manner. Inspired by the work of Oparin and Bungenberg-de Jong2, we show that this problem in prebiotic chemistry can be solved by condensing the oxidizing agent into coacervate droplets.
Coacervates are liquid condensates, rich in water, that lack a surrounding membrane. They are usually formed from large molecules including polymers, proteins, and nucleotides 3, although recently more prebiotically plausible coacervates made with short peptides or common metabolites found across all forms of life have been reported4,5. Other multivalent molecules can be concentrated by several orders of magnitude in coacervate droplets, and their effective concentration can reach hundreds of mM or even M levels. Thus, in our recent paper in Nature Communication6, we hypothesized to make complex coacervates from organic and inorganic ferricyanide, which makes the coacervates redox responsive and allows chemical control over the assembly/disassembly.
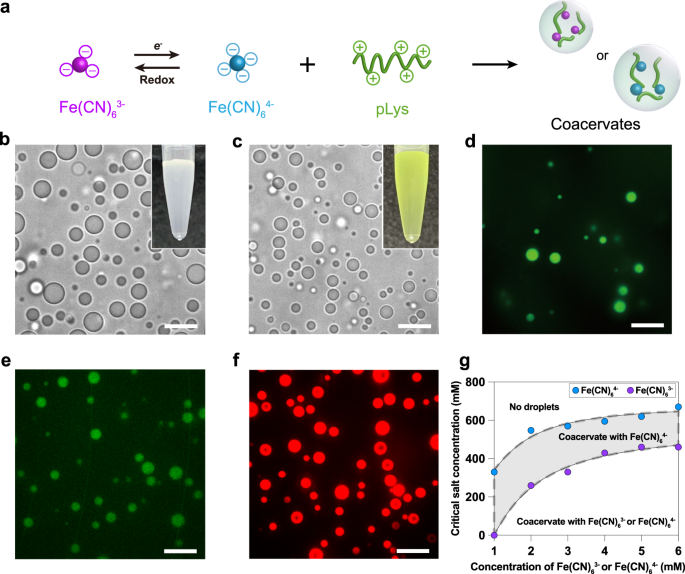
We used short cationic peptides to induce phase separation of ferrocyanide and its reduced (Fe(CN)63− and Fe(CN)64−), and spontaneously a turbid dispersion was observed (Fig. 1) on mixing with several different peptides motifs ((Lys)10, (Lys)20,(Lys(Me)3)20, (Lys)30, (Lys(Me)3)30, poly-L-lysine (pLys) and (Arg)10). This turbid dispersion solution contains complex coacervate droplets when observed by the optical microscope. The mixture of inorganic tetravalent motif Fe(CN)64- with polycations is white turbid and formed complex coacervates while the mixture of trivalent motif Fe(CN)63- with polycations is yellowish homogeneous transparent solution. By using different oxidizing agents, the white turbid solution can be converted into a transparent yellowish solution, indicating that coacervates are dissolved. Then, by using different reducing agents, like GSH and NADH, the solution turned turbid again and coacervates reemerged. This shows the complete control over the assembly and disassembly of coacervates through redox chemistry.
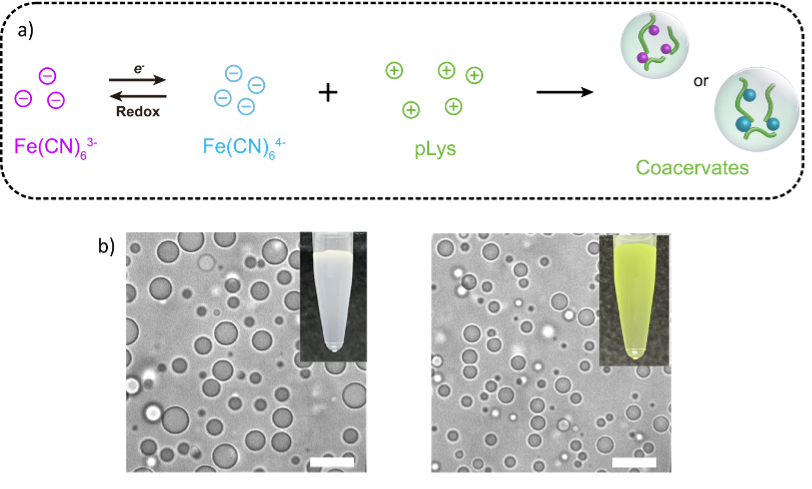
Fig.1. General scheme for the synthesis of organic-inorganic coacervates (a), optical microscope images of coacervates formed from ferrocyanide and its reduced form with polycations and insets are the pictorial representation of turbid solutions. Scale bars, 10 μm.
Interestingly, the size of coacervates is tunable depending on the different ratios of the components used and pre-treated slides that make these compartments a promising candidate as a microreactor. Thus, after the sequestration of relevant small molecules, we chose the amino acids for amide bond formation and acylation reaction inside coacervates. The partitioning coefficients confirm that amino acids are entrapped within the condensed phase of coacervates, thus, adding prebiotically relevant amino acids and α-amidothioacids in stoichiometric amounts to coacervates results in the formation of dipeptides through amide/peptide bond in the redox-responsive catalytic reservoir. (Fig. 2)
Moreover, a ligation reaction was carried out with two types of amino acids instead of one that can react with the α-amidothioacids. Whereas both Gly and Glu can separately be ligated to Ac-Gly-SH with yields up to 90%, only Gly reacted to form the ligation product Ac-Gly-Gly-OH in a 9-fold excess over Ac-Gly-Glu-OH, when a mixture of Gly/Glu (1:1) was added. Other mixtures of amino acids showed similar preferential product formation. This shows that the coacervate environment imposes a selection pressure that results in the preferential incorporation of certain amino acids over others. This selectivity is driven by a combination of factors, including local concentration, intrinsic reactivities, and interactions with the coacervate matrix. This finding sheds light on how early chemical environments might have influenced the composition of primitive peptides.
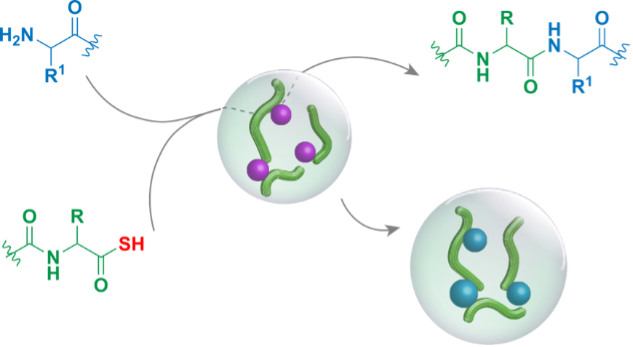
Fig. 2. Peptide bond formation inside the organic-inorganic coacervate droplets.
These complex coacervates are also able host to thiol-containing building blocks that can self-assemble to form nanofibers and interestingly we observed the self-assembly of nanofibers resembling a cytoskeletal network inside the coacervates. (Fig.3) This hints at the potential role of these coacervates in the early organization of molecular structures reminiscent of cellular components.
In summary, the study provides compelling evidence for the potential of Fe(CN)63--based coacervates as protocellular oxidizing hubs in prebiotic chemistry. By demonstrating their ability to drive essential prebiotic reactions, such as peptide bond formation and oxidation of metabolites, the research opens new avenues for understanding the chemical processes that may have led to the emergence of life on Earth.
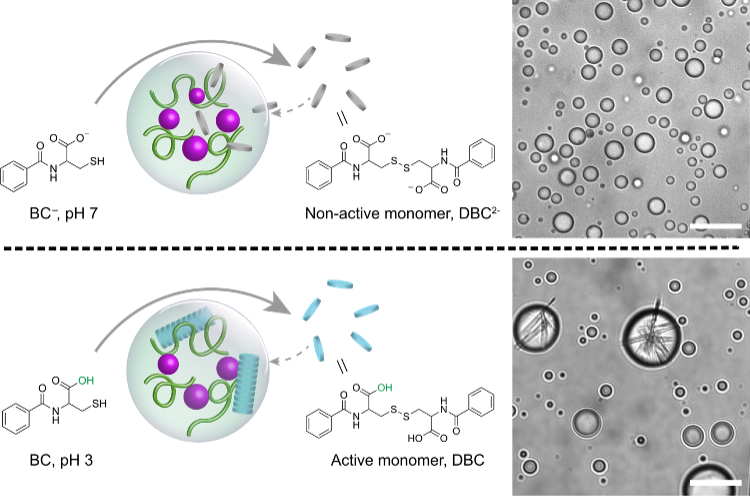
Fig. 3. Nanofiber formation through self-assembly inside coacervate droplets. Scale bars, 10 μm.
References
- Keefe, A. D. & Miller, S. L. Was ferrocyanide a prebiotic reagent? Orig. Life Evol. Biosphere 26, 111–129 (1996)
- Bungenberg de Jong, H. G. & Kruyt, H. Coacervation (Partial miscibility in colloid systems). Proc. K. Ned. Akad. Law. 32, 849–856 (1929)
- Abbas, M., Lipinski, W. P., Wang, J. & Spruijt, E. Peptide-based coacervates as biomimetic protocells. Chem. Soc. Rev. 50, 3690–3705 (2021)
- Smokers, I. B. A., van Haren, M. H. I., Lu, T. & Spruijt, E. Complex coacervation and compartmentalized conversion of prebiotically relevant metabolites. ChemSystemsChem 4, e202200004 (2022)
- Cakmak, F. P., Choi, S., Meyer, M. O., Bevilacqua, P. C. & Keating, C. D. Prebiotically-relevant low polyion multivalency can improve functionality of membraneless compartments. Nat. Commun. 11, 5949 (2020)
- Wang J, Abbas M, Wang J, Spruijt E. Selective amide bond formation in redox-active coacervate protocells. Commun. 14, 8492 (2023)
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in