Photocatalytic H2O2 Production with both O2 Reduction and Water Oxidation
Published in Chemistry
Hydrogen peroxide (H2O2) is an important chemical for a sustainable society, widely used in the field of bleaching, green chemical synthesis, and wastewater treatment.1 It can also be used as a liquid fuel alternative to H2 or fossil fuels in fuel cells.2 With the increasing demand, the global market for H2O2 is expected to grow to 5.7 million tons by 2027.3 Up to now, the production of H2O2 mainly relies on an unsustainable anthraquinone (AO) method, such as using of noble metal Pd-based catalysts, consuming large amounts of energy, and generating large amounts of toxic by-products.4 Photocatalytic H2O2 production is considered as an alternative to the AO method because the technology, ideally, consumes only O2, H2O, and sunlight, with the advantage of environmental friendliness and economic feasibility.5 Currently, the effectiveness of the photocatalytic H2O2 production is limited by three main issues, including the usage of additional organic sacrificial reagents in most H2O2 production systems,6 the less clarity of the pathway of photogenerated holes in the few available systems enabling H2O2 production from only H2O and O2,7 and the insufficient wavelength response only in the visible region (< ca. 600 nm, 31% of solar energy).7,8
In this work, we report self-assembled tetrakis(4-carboxyphenyl)porphyrin (SA-TCPP) supramolecular photocatalysts effectively produce H2O2 from H2O and O2 through dual pathways of oxygen reduction and thermal-assistant water oxidation. In particular, after SA-TCPP is irradiated at room temperature, we find photogenerated electrons reduce O2 adsorbed by the pyrrole N-H ring, while photogenerated holes oxidize carboxylic group (-COOH) to peroxy acid group (-CO3H) intermediates. Peroxy acid intermediates are known to be unstable, and input thermal energy is potential to promote them to produce H2O2. As a result, we perform the reaction at 353 K, the H2O2 production is significantly improved to achieve the rate ca. 0.66 mM h-1 for the first hour, and the total accumulation of 2.51 mM has been realized for the 4 h experiment. By comparison, SA-TCPP at room temperature produces few H2O2 (0.22 mM after 4 h). The stability of the photocatalyst can be preserved for 80 h experiment, and every 20 h accumulates ca. 50 mM of H2O2. By optimizing the concentration of the SA-TCPP photocatalysts, the total H2O2 accumulation of 6.90 mM has been realized for the 4 h experiment. The quantum efficiency is ca. 14.9% at 420 nm and ca. 1.1% at 940 nm (Figure 1). The solar energy to chemical conversion efficiency (SCC) reaches ca. 1.2% at 328 K irradiated and heated with simulated sunlight. A lab-made flow reactor has been applied to isolate the produced H2O2, and an evaporation dish has been designed to concentrate the produced H2O2. By this method, H2O2 can be accumulated to the concentration of ca. 1.1 wt%, which is close to the typical concentration for H2O2 in the household (ca. 3.0 wt%).
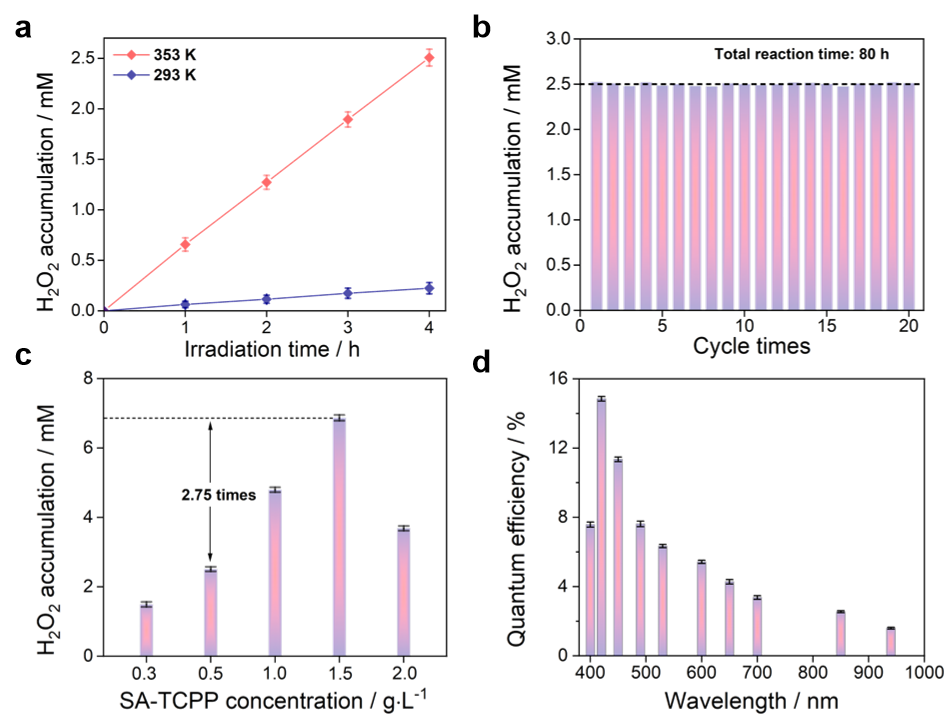
Figure 1. H2O2 production performance on SA-TCPP supramolecular photocatalysts. Sln. 50 mL H2O, Temp. 353 K, Cat. 0.5 g/L, O2 bubbling. Light source: Xe lamp with a 420 nm cut-off filter. a, H2O2 production on SA-TCPP supramolecule at 353 K and 293 K, respectively, plotted as a function of irradiation time. b, Stability for H2O2 production of SA-TCPP supramolecule. c, H2O2 production with different amounts of SA-TCPP supramolecule. d, Quantum efficiency on SA-TCPP supramolecular photocatalysts with different bandpass filters. (Cat. 1.5 g/L).
The hole-induced H2O2 production pathway is directly demonstrated by TOF-MS, NMR and isotopic experiments (Figure 2). The TOF-MS spectra provide structure information on the produced peroxy acid intermediates. After the reaction at room temperature, four peaks representing the intermediate emerged, with m/z of 805.2, 806.2, 807.2, and 808.2. The increment between the intermediate and SA-TCPP (789.2, 790.2, 791.2, and 792.2)is 16, indicating the introduction of 16O to the -COOH groups. The results of 13C NMR spectra further illustrate that -CO3H groups tend to accumulate at a lower temperature (293 K), while they decompose at a higher temperature (353 K). The isotopic experiments with H218O indicates the photogenerated electrons and holes used for H2O2 production are 1:1.
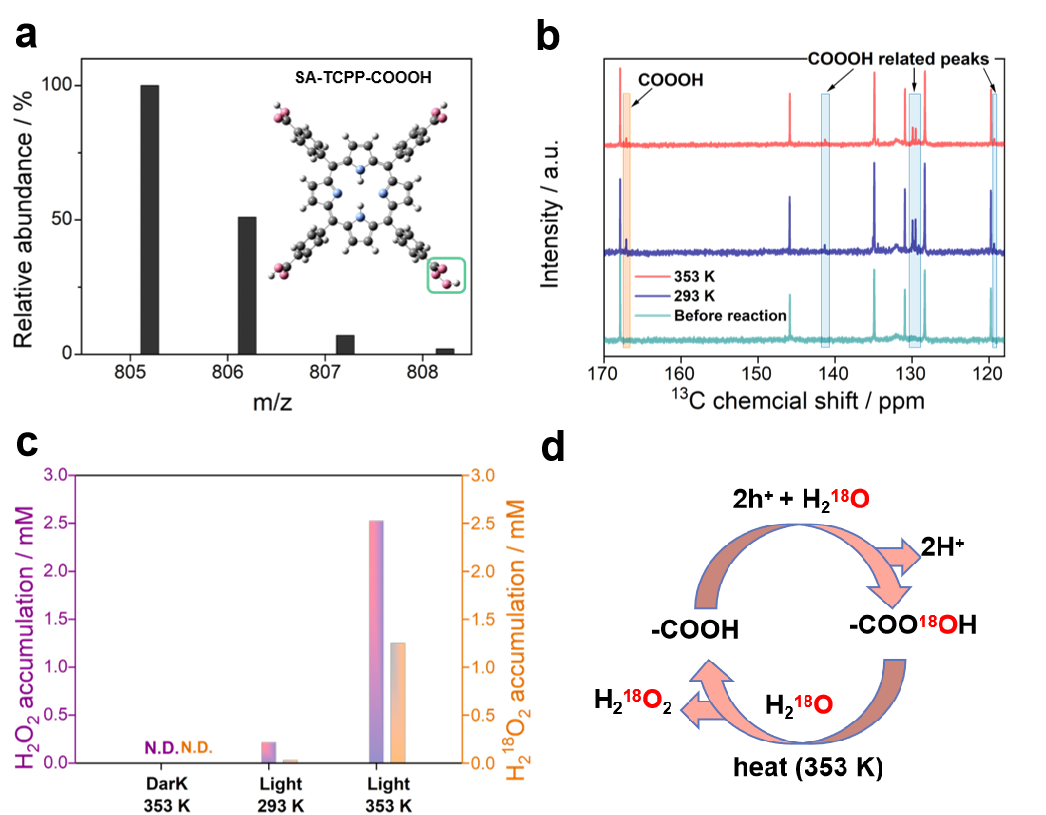
Figure 2. Peroxy intermediates generation at 293 K and 353 K. a, The molecular ion peak obtained from the ESI(-)-TOF-MS spectrum for SA-TCPP-COOOH. Inset is the molecular structure of SA-TCPP-COOOH. O: Pink, C: Grey, N: blue, H: white, and green rectangle: the peroxy carboxylic acid group (-CO3H). b, 13 C NMR for H2O2 production on SA-TCPP supramolecular photocatalyst after reaction at 293 K and 353 K. c, Isotopic experiments with H218O for H2O2 production on SA-TCPP supramolecular photocatalyst after reaction at 293 K and 353 K. d, The proposed schematics for H2O2 production on SA-TCPP by holes according to the isotopic experiments.
Besides 2e- O2 reduction to produce H2O2 in SA-TCPP, our work highlights a hole-induced H2O2 production process, which involves the photoconversion of -COOH to -CO3H groups on SA-TCPP supramolecular photocatalyst, followed by thermal decomposition. The work not only provides a material platform for effective H2O2 production from solar energy but also provides guidance for designing suitable organic photocatalysts to achieve higher efficiency.
For more details, please check out our paper “H2O2 generation from O2 and H2O on a near-IR absorbing porphyrin supramolecular photocatalyst” in Nature Energy (https://doi.org/10.1038/s41560-023-01218-7).
References
- Campos-Martin, J. M.et al. Hydrogen peroxide synthesis: an outlook beyond the anthraquinone process. Chem. Int. Ed. 45, 6962-6984 (2006).
- Xue, et al. Electrochemical and photoelectrochemical water oxidation for hydrogen peroxide production. Angew. Chem. Int. Ed. 60, 10469-10480 (2021).
- Kondo, et al.Design of metal-organic framework catalysts for photocatalytic hydrogen peroxide production. Chem 8, 2924-2938 (2022).
- Chen, D. et al.Covalent organic frameworks containing dual O2 reduction centers for overall photosynthetic hydrogen peroxide production. Angew. Chem. Int. Ed. 62, e202217479 (2023).
- Moon, G.-h. et al.Eco-friendly photochemical production of H2O2 through O2 reduction over carbon nitride frameworks incorporated with multiple heteroelements. ACS Catal. 7, 2886-2895 (2017).
- Hou, H.et al. Production of hydrogen peroxide by photocatalytic processes. Chem. Int. Ed. 59, 17356-17376, (2020).
- Zhi, Q.et al. Piperazine-linked metalphthalocyanine frameworks for highly efficient visible-light-driven H2O2 J. Am. Chem. Soc. 144, 21328−21336 (2022).
- Kou, M.et al. Molecularly engineered covalent organic frameworks for hydrogen peroxide p Angew. Chem. Ind. Ed. 61, e202200413 (2022).
Follow the Topic
-
Nature Energy

Publishing monthly, this journal is dedicated to exploring all aspects of this on-going discussion, from the generation and storage of energy, to its distribution and management, the needs and demands of the different actors, and the impacts that energy technologies and policies have on societies.
Related Collections
With Collections, you can get published faster and increase your visibility.
Microgrids and Distributed Energy Systems
Publishing Model: Hybrid
Deadline: Mar 31, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in