Reaction Sites on Ferrihydrite
Published in Chemistry

This work (see paper here) begun over 10 years ago in a period where we were exploring the relationship between crystal habits of nanominerals and the reactive OH functional groups they expose [e.g. 1-2]. Inspired by the catalytic alumina literature [3], we used vibrational spectroscopy to identify OH groups of various coordination and hydrogen bonding environments on iron (oxyhydr)oxides nanoparticles. This was to set the stage for our later work tracking reactions on distinct crystal faces of multifaceted nanominerals, including water film growth mechanisms [4-5], which are needed in the study of mineral-driven atmospheric and geochemical processes on Earth.
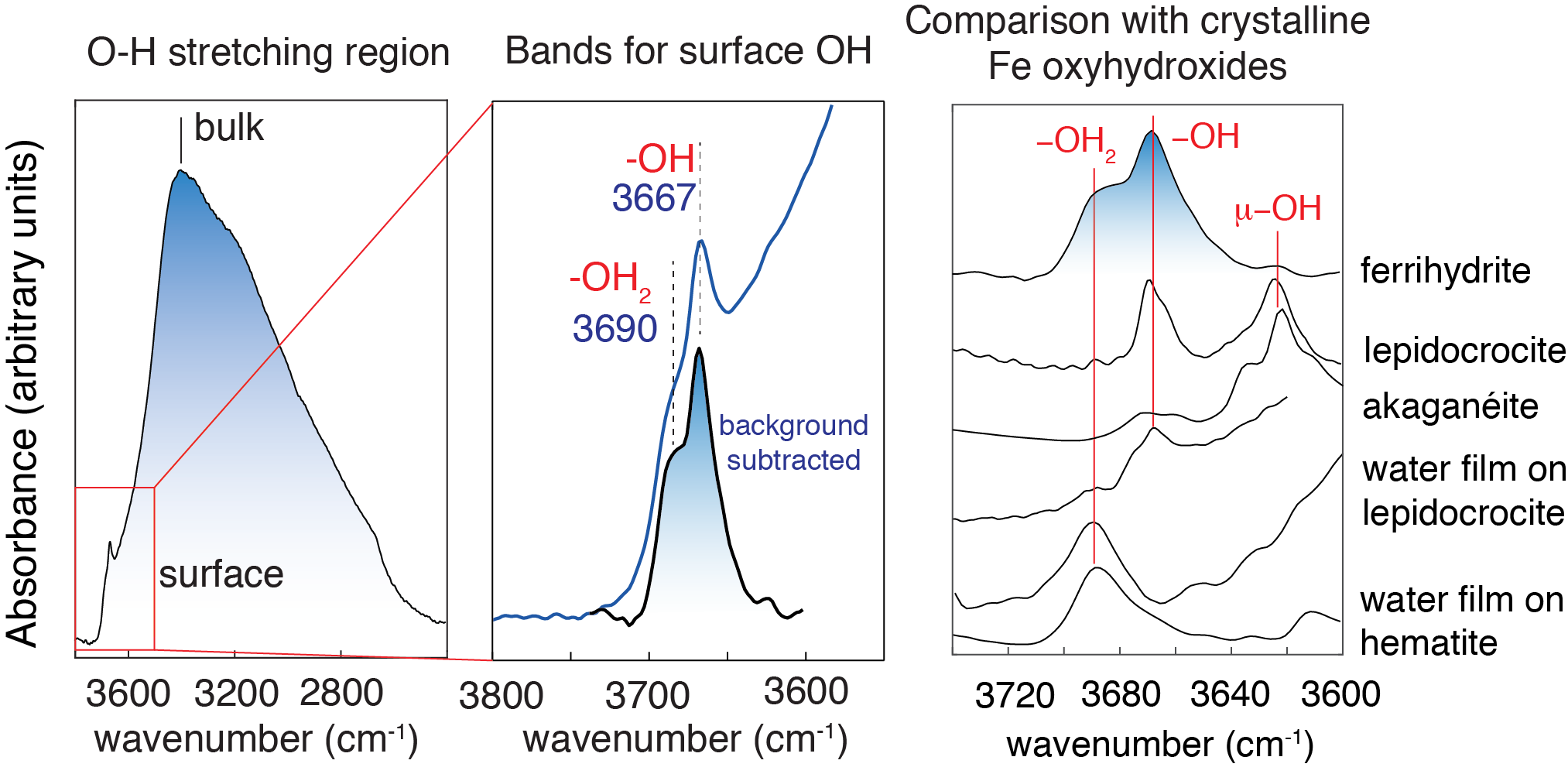
While we had mostly focused our work on crystalline nanominerals, we also took the opportunity in 2011 to detect OH groups on ferrihydrite, which forms spheroidal nanoparticles of variable crystallinity. Exploring these groups on ferrihydrite was motivated by the tremendous roles this mineral plays in nature. We found high densities of the reactive singly-coordinated (Fe-OH) sites, and their spectral profiles were highly comparable to those of more crystalline iron (oxy)hydroxide phases. While I had in those days co-authored a paper on the structure of a ferrimagnetic form of ferrihydrite [6], ongoing debates over competing structural models — and uncertainties over which crystallographic faces could best describe ferrihydrite surfaces — compelled us to leave this work aside until we knew more about the topic.
And so, we let those data sit on the back burner for a longer period than we had initially imagined. Co-author Xiaowei Song went on to complete her Ph.D. in my group in 2013, spent three years as a postdoctoral fellow at the Fritz-Haber institute in Berlin, then finally joined the R&D team of IKEA of Sweden.
Only years later, in 2019, did we finally decide to re-explore our data after reading an excellent series of papers [e.g. 7-8] by Tjisse Hiemstra (Wageningen University) who had proposed OH populations based on an idealised representation of ferrihydrite nanoparticles. The model envisions nanoparticles composed of (i) a defect-free core consisted of the low OH-bearing structure of Michel et al. [9] (Fe5O8H) and (ii) crystallographically-oriented surfaces of greater OH densities (Fe5O8H + n H2O) but with depleted densities of two types of Fe sites. Despite its simplifications, this Surface Depletion (SD) model opened possibilities for rethinking the reactivity of ferrihydrite under a new light.
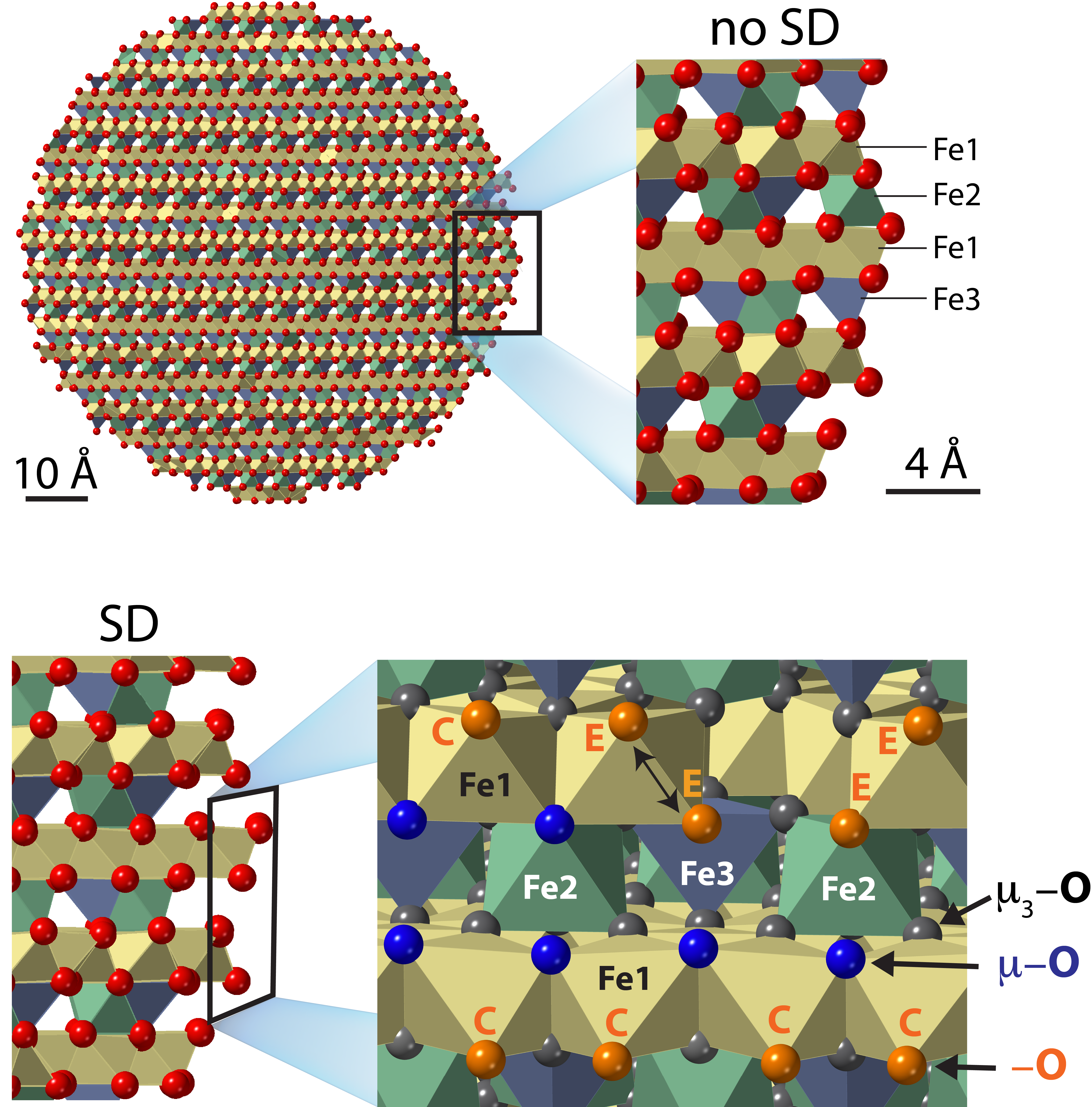 Enthused by these developments, we took an another look at our data and found that the dominance of a poorly hydrogen-bonded singly-coordinated group aligned with this model. However, skeptical that any crystallographic faces should predominantly be used to represent ferrihydrite surfaces, we developed a computer code that explored the composition of a wide variety of particles of spheroidal morphology. Additionally, we performed the first Molecular Dynamics simulations of single nanometric ferrihydrite particles of up to 10 nm in diameter. Our simulations suggest that reactive surface OH groups are chiefly disposed along rows at edge of sheets of Fe octahedra, and that the O-H bonds are generally free (not hydrogen bonded). Based on comparisons with crystalline materials, we find that these latter results align with the vibrational spectroscopic response of ferrihydrite.
Enthused by these developments, we took an another look at our data and found that the dominance of a poorly hydrogen-bonded singly-coordinated group aligned with this model. However, skeptical that any crystallographic faces should predominantly be used to represent ferrihydrite surfaces, we developed a computer code that explored the composition of a wide variety of particles of spheroidal morphology. Additionally, we performed the first Molecular Dynamics simulations of single nanometric ferrihydrite particles of up to 10 nm in diameter. Our simulations suggest that reactive surface OH groups are chiefly disposed along rows at edge of sheets of Fe octahedra, and that the O-H bonds are generally free (not hydrogen bonded). Based on comparisons with crystalline materials, we find that these latter results align with the vibrational spectroscopic response of ferrihydrite.
This work is now allowing us to explore reactions with environmental gases (e.g. H2O, CO2, CH4, NOx, SOx) and (photo-sensitive) organics to better understand the crucial roles ferrihydrite nanoparticles play in Earth's soils and atmosphere.
References
1. Song X., Boily J.-F. Phys. Chem. Chem. Phys. 14, 2579-2586 (2012).
2. Song X., Boily J.- F. Chem. Phys. Lett. (Frontiers) 560,1-9 (2013).
3. Busca G. Catal. Today 226, 2–13 (2014).
4. Boily J.-F., Yesilbas M. Munshi MMU, Baiqing L, Trushkina Y, Salazar-Alvarez G., Langmuir 31, 13127–13137 (2015).
5. Boily J.-F. , Fu L., Tuladhar A., Lu Z., Legg B., Wang M., Wang H.-F. J. Colloid Interface Sci. 555, 810-817 (2019).
6. Michel FM, Barrón V, TorrentJ, MoralesMP, Serna CJ, BoilyJ-F, Liu Q, Ambrosini A, Cismasu CA, Brown Jr. GA. Proc Nat. Acad Sci. 107, 2787-2792 (2010).
7. Hiemstra T. Geochim. Cosmochim. Acta 105, 316–325 (2013).
8. Hiemstra T. Environ. Sci. Nano 5, 752–764 (2018).
9. Michel F.M., Ehm L., Antao S.M., Lee P.L., Chupas P.J., Liu G., Strongin D.G., Schoonen M.A.A., Phillips B.L., Parise J.B. Science 316, 1726–1729 (2007).
For more information about our research please visit our webpage
Follow the Topic
-
Communications Chemistry

An open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of the chemical sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Experimental and computational methodology in structural biology
Publishing Model: Open Access
Deadline: Apr 30, 2026
Advances in Asymmetric Catalysis for Organic Chemistry
Publishing Model: Open Access
Deadline: Mar 31, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in