The Immuno-Storm Chip: Counting Single Cytokine Molecules in Blood before the Immune System goes Haywire in Cancer Treatment and Infectious Disease
Published in Bioengineering & Biotechnology

From treating cancers with heat and “cutting it out” as practised by ancient Greeks all the way to precisely irradiate the tumour with X-rays and specifically inhibit aberrant cancer signalling pathways as we treat cancer today, the story of our fight against this severe disease is truly remarkable. In the last decades, we have seen a paradigm change in cancer therapy as technology progressed and allowed us to better understand, and importantly, better treat cancer based on a patient-specific molecular profile. Precision oncology was born at the right moment. Precision oncology is now at a stage where it not only focuses on the tumour, but also how the patient’s immune system responds to the treatment. This has given rise to immunotherapy, where the inhibition of immune checkpoint receptors aims to re-activate the immune system and eliminate the tumour. Undoubtedly, immunotherapy has saved many lives with the unique advantage of long-lasting responses. Yet, the therapy that stimulates the immune system to attack the cancer has to be finely adjusted to the patient: an over-stimulation triggers severe immune-related adverse events (irAEs), which is when the immune system goes 'haywire' and starts attacking the patient’s own body. irAEs can be mild to severe with symptoms of minor skin rashes to systemic inflammation. Unfortunately, irAEs occur in up to 80% of all cancer patients treated with immunotherapy, often leading to an early cessation of treatment and even be life-threatening for certain patients.
Cytokine are small signalling molecules that are involved in the immune response and have been associated with irAEs in cancer patients. However, the concentration of some cytokines found in circulation is extremely low, challenging many conventional immunoassay. Knowing that irAEs can be life threatening for some patients, we were motivated to develop a ‘nano-strategy’ to detect patient-specific cytokines signatures to indicate irAE occurrence. If successful, immunotherapy could be adjusted before the onset of irAEs, and thus the patients would still benefit from the treatment.
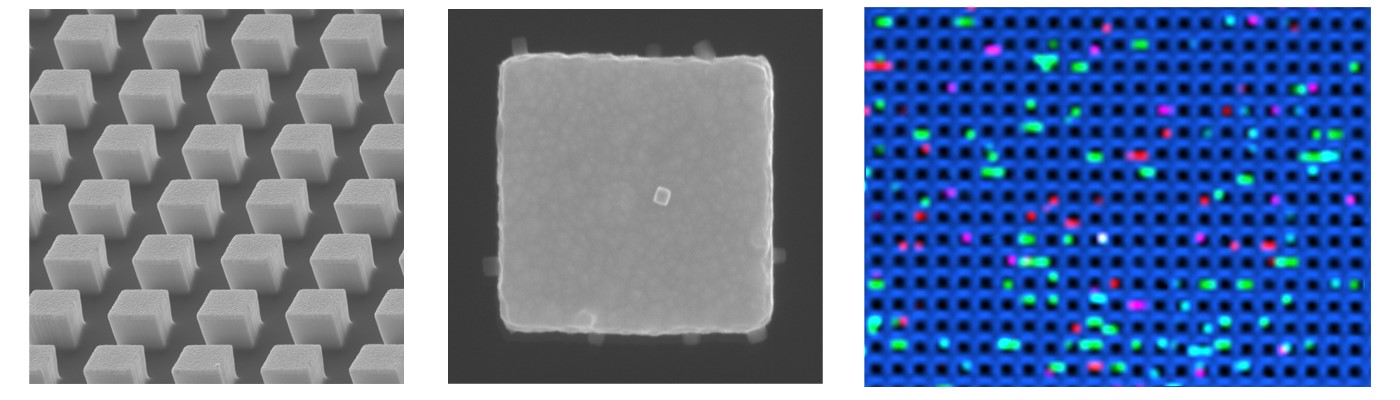
In our group at the Centre for Personalised Nanomedicine, we prepare and study nanomaterials to deliver diagnostic strategies to support precision medicine and oncology. We synthesised gold-silver alloy nanobox particles that showed single particle activity in surface-enhanced Raman scattering (SERS). These super-bright nanoboxes would be a perfect match for trace cytokine detection, where it should be possible to reflect and detect single molecules. Similar to digital droplet polymerase chain reaction (ddPCR), we aimed to establish a digital counting assay. But there was a ‘but’: Having super-bright nanoboxes was important, but we still needed a platform to accommodate single nanoboxes and single cytokine molecules. To make that happen, our group started to use electron-beam lithography, which allows to precisely fabricate nano-structures. Like the droplet compartments in ddPCR, we thought we can nanofabricate tiny compartments to accommodate a single molecule and single nanobox. Instead of a droplet, we created a nanopillar array, or a forest of 250,000 pillars per square millimetre, which was functionalised with cytokine-capturing antibodies. To make sure that we could count cytokines, we diluted the sample to follow Poisson distribution. At this stage we had a technology, but no patient samples to test the technology. We reached out to melanoma experts and clinical researcher at the Olivia Newton-John Cancer Research Institute in Melbourne (Australia) to find longitudinally, immunotherapy-treated melanoma patients. Lucky us we got access to such a precious patient cohort. As we report in the article, we found that the elevation of cytokine concentration during immunotherapy was correlated with the onset of irAEs, although the study needs to be extended to a larger sample cohort in the future to confirm the results. We were very delighted with this promising finding as our ultimate aim is to develop diagnostic nanotechnologies to improve patient lives.
Cytokines are not only involved in irAEs in cancer, but also in other diseases where the immune system goes ‘haywire’ and significantly worsen the patients’ health. Prominent examples of this include acute COVID-19, long haul COVID-19 disease and also Sepsis. For reasons we don’t yet totally understand, whether it’s due to genetics or immune history, around 1% of COVID-19 patients get a crazy cytokine storm. These patients experience extensive tissue damage – particularly in the lungs - and have a poor prognosis. It now seems clear that many COVID-19 deaths are caused by this mechanism, and that many of the multiple site tissue damage affects experienced by COVID-19 long haul patients can also be attributed to this phenomenon.
Whether in a cancer treatment setting or when monitoring infectious diseases, our Nanopillar (“Immuno-storm Chip”) technology could provide critical medical information that guides important clinical decisions. For example, it would allow hospitals to focus resources on patients at high-risk of a cytokine storm, while low-risk patients are monitored from home. Critically, it could inform doctors to begin, or to ease off treatments, by accurately monitoring the patient’s immune response before it goes crazy. Detection of the detailed cytokine signature for vulnerable acute and long haul COVID-19 patients with the Immuno-storm chip could also be used to personalise the therapy of these patients, tuned in to alleviate their specific excessive immune system response. Future clinical studies will be utilised to address these issues.
This Behind the Paper article was written by Junrong Li, Alain Wuethrich, and Matt Trau.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in