The safety and efficacy of bispecific T-cell engagers (TCEs) in patients with glioma
Published in Cancer and General & Internal Medicine
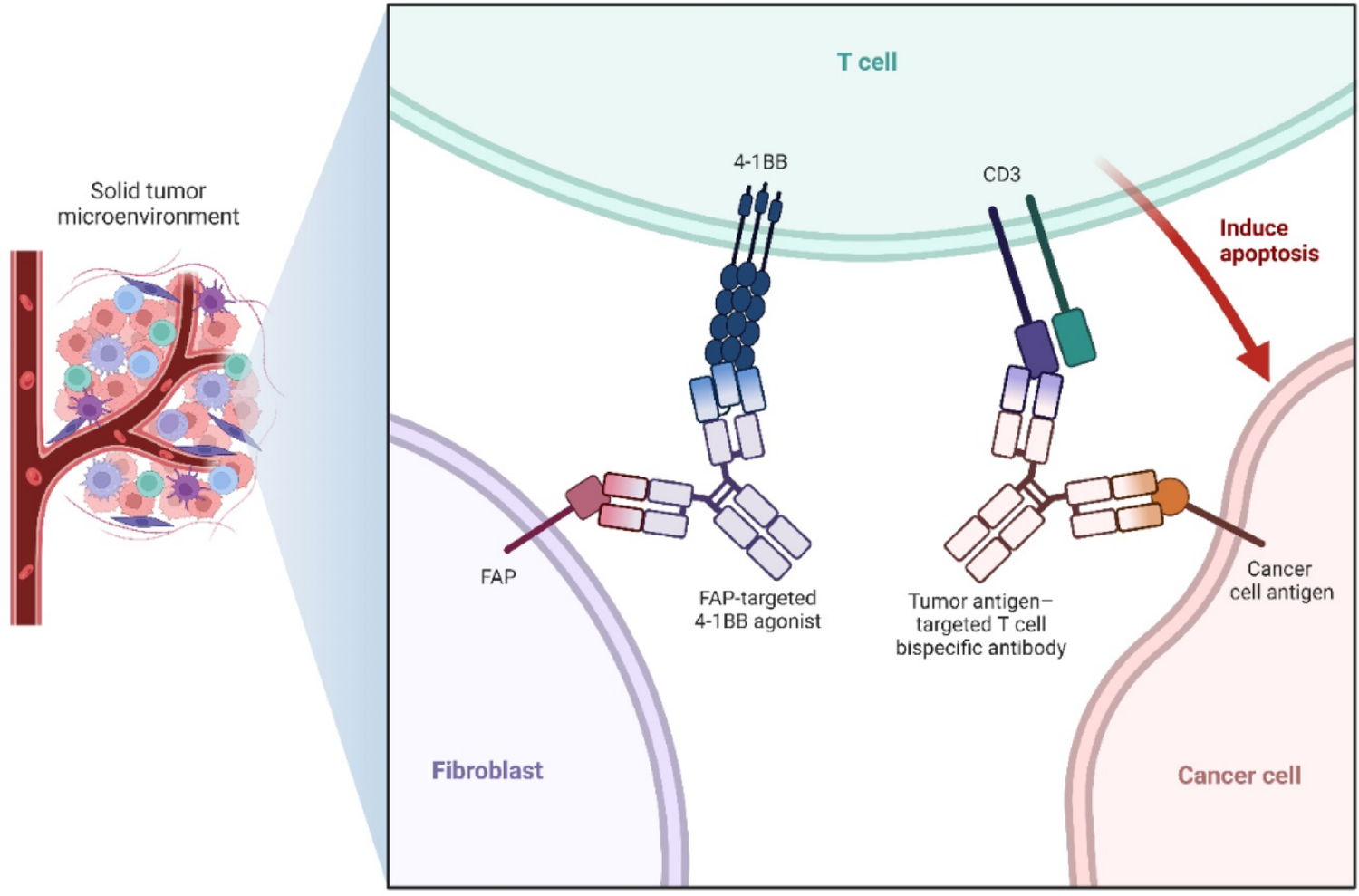
Among the most aggressive and resistant tumors of the central nervous system, glioblastoma (GBM) has a poor prognosis and few available treatments. Because of the tumor’s infiltrative nature, immunosuppressive environment, and resistance mechanisms, traditional treatments such as radiotherapy, chemotherapy, and surgery offer only modest survival benefits. Bispecific T-cell engagers (TCEs) have shown promising preclinical and early clinical results, and immunotherapy has become a feasible strategy.
TCEs efficiently promote antigen evasion and strong tumor lysis by directing cytotoxic T lymphocytes (CTLs) to tumor-associated antigens (TAA) such as the EGFRvIII ligands IL-13Rα2, Fn14, and NKG2D ligands (NKG2DLs). Although phase I clinical studies with AMG 596 have shown acceptable safety profiles and early indications of efficacy, preclinical mice have demonstrated prolonged longevity.
However, challenges still exist, including the short half-life of TCEs molecules, limited T-cell infiltration, antigen heterogeneity, and the risk of neurotoxicity or cytokine release syndrome (CRS). Promising developments include novel approaches such as multivalent targeting, DNA-encoded or cell-delivered TCEs, and combinations with immune checkpoint inhibitors (ICIs) or CAR-T cells. With an emphasis on its integration into multimodal treatment approaches, this review highlights the safety, effectiveness, and potential uses of TCEs immunotherapy for gliomas.
See here for the article: https://link.springer.com/article/10.1007/s10238-026-02057-yIF: 3.5 Q2
-Kamyar Bagheri
Follow the Topic
Ask the Editor – Inflammation, Metastasis, Cancer Microenvironment and Tumour Immunology
Got a question for the editor about inflammation, metastasis, or tumour immunology? Ask it here!
Continue reading announcementRelated Collections
With Collections, you can get published faster and increase your visibility.
Clinical Practice
Publishing Model: Open Access
Deadline: Ongoing
Myeloproliferative Neoplasms: Pathophysiology and Therapeutic Advancement
Myeloproliferative neoplasms (MPNs) encompass a diverse group of hematological malignancies characterized by the overproduction of blood cells due to dysregulation of hematopoiesis. This Collection seeks to explore the pathophysiological mechanisms underlying conditions such as polycythemia vera, essential thrombocythemia, and myelofibrosis. Understanding these disorders at the molecular and cellular levels is crucial for developing more effective diagnostic and therapeutic strategies, particularly as our knowledge of the genetic and epigenetic factors involved in MPNs continues to expand.
The significance of ongoing research in this field is highlighted by recent advances in targeted therapies and molecular diagnostics, which have the potential to transform patient outcomes. Innovations such as gene expression profiling and improved understanding of platelet biology have opened new avenues for treatment, enabling personalized medicine approaches that cater to individual patient needs. As the landscape of MPNs management evolves, it becomes increasingly vital to address challenges related to leukemia transformation and disease progression, ensuring that patients receive optimal care.
Future research in myeloproliferative neoplasms may lead to the identification of novel therapeutic targets and biomarkers that further enhance our ability to predict disease outcomes and tailor treatments. Additionally, advancements in gene editing technologies and immunotherapy could pave the way for groundbreaking strategies in disease management. By continuing to investigate the underlying mechanisms of these disorders, we can aspire to improve quality of life and survival rates for patients affected by MPNs.
Researchers are encouraged to submit their work to this Collection, which aims to showcase innovative research on the pathophysiology and therapeutic advancements in myeloproliferative neoplasms. We welcome studies that contribute to a deeper understanding of these conditions and explore new avenues for improved diagnosis and treatment.
Publishing Model: Open Access
Deadline: Aug 31, 2026

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in