The surprising lability of zeolites
Published in Chemistry
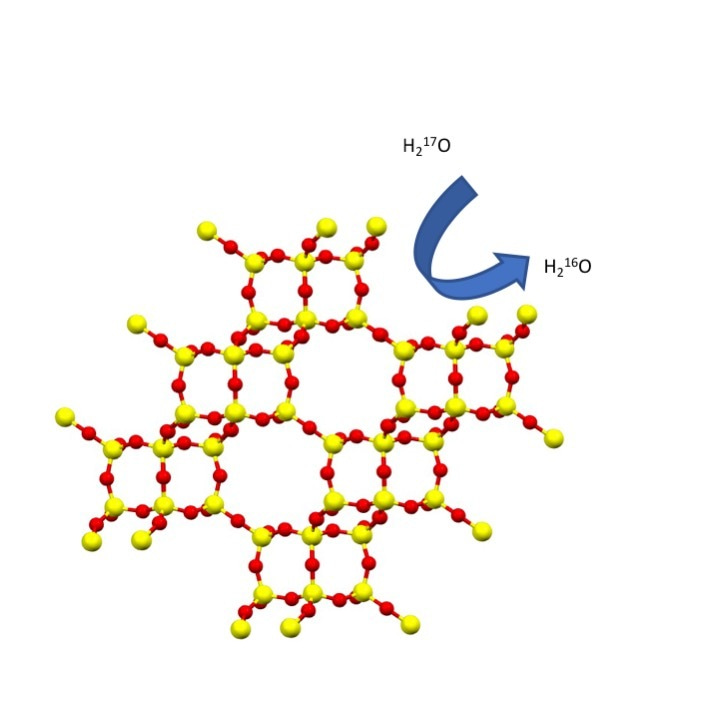
Zeolites are some of the most important industrial chemicals known, with applications across many forms of catalysis, ion exchange and even medicine. The defining feature of zeolites is the interplay between their porous architectures and the chemistry of the internal surfaces of the pores and channels. This leads directly to most of the successful applications of these important solids.
The picture we generally have of zeolites under ambient conditions is that they are static materials (apart from, of course, vibrational excitations), with windows into pores that control access to the internal surface of the solid. This picture is not a surprising one given that many zeolites are naturally occurring minerals and so are often described as inert. However, in a recent Nature Communications publication we show that even at room temperature aluminosilicate zeolites rapidly exchange oxygen atoms when in contact with liquid water.
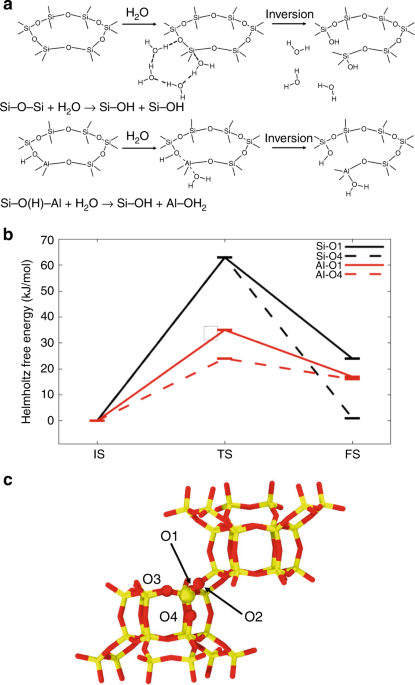
Computationally, we showed that there are credible mechanisms that show how the first steps of hydrolysis could occur relatively easily. The calculations used a technique called ab initio molecular dynamics (AIMD). The predicted mechanisms differ from those that have been proposed previously in that they involve a chain of water molecules along which a proton can be transferred, leading to more stable hydrolysis products than would be the case for other mechanisms.
Experimentally, we then demonstrated that oxygen is readily exchanged into a zeolite by contacting the material with oxygen-17 enriched water. Given that 17O is an NMR active nucleus, we can then follow the process using solid-state MAS NMR. The experiments show that even after ten minutes, resonances due to both Si – 17O – Al and Si – 17O – Si are clearly visible in triple-quantum MAS NMR experiments, indicating just how surprisingly labile zeolites are in contact with water. A time study indicates that isotopic exchange into the Si – O – Al bonds is faster than that into the Si – O – Si. This agrees well with the predictions from the computations. There is no degradation of zeolite visible in either X-ray diffraction or NMR studies, but nevertheless there is fast isotopic enrichment.
The big question is: how important is this observation of fast room temperature lability in aluminosilicate zeolites? The first thing we can say is that this has been a surprise to others in the zeolite community who didn’t expect to see this result. It also raises questions about the low temperature behaviour of zeolites, and in particular how important the bond-breaking/bond-making processes are in determining what molecules can access the internal space of the solid from aqueous solutions. Further computational and experimental studies will help us to determine the answer.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in