Unlocking Chemical Trends: A Simple Model Explains Non-Intuitive Reactivity and Guides Technological Solutions
Published in Chemistry
In their work, Artem R. Oganov and Maksim G. Kostenko from Skoltech introduce a refined chemical model where each element is characterized by just two numbers: an electronegativity (X) and a chemical mismatch parameter (Y). The propensity for two elements (A and B) to form a stable compound is distilled into a single "interaction parameter" Q:
Q = - [X(A) - X(B)]² + [Y(A) - Y(B)]²
Q is proportional to the enthalpy of formation of a binary AB compound; negative Q favors compound formation; a positive Q indicates instability. This formula generalizes ideas from earlier models by Pauling and Miedema, extending reliable predictions across the entire periodic table and offering a powerful framework to explain surprising chemical behavior and address practical technological challenges.
Key Insights and Practical Utility:
1. Explaining Non-Intuitive Phenomena: The model's primary power lies in rationalizing counterintuitive chemical trends. It clearly shows why highly electropositive alkali metals react with surprisingly few elements, while so-called "inert" noble metals like platinum form compounds with many. This is due to the critical balance between the stabilizing electronegativity difference and a destabilizing term governed by the mismatch parameter Y, which is extreme for alkali metals.
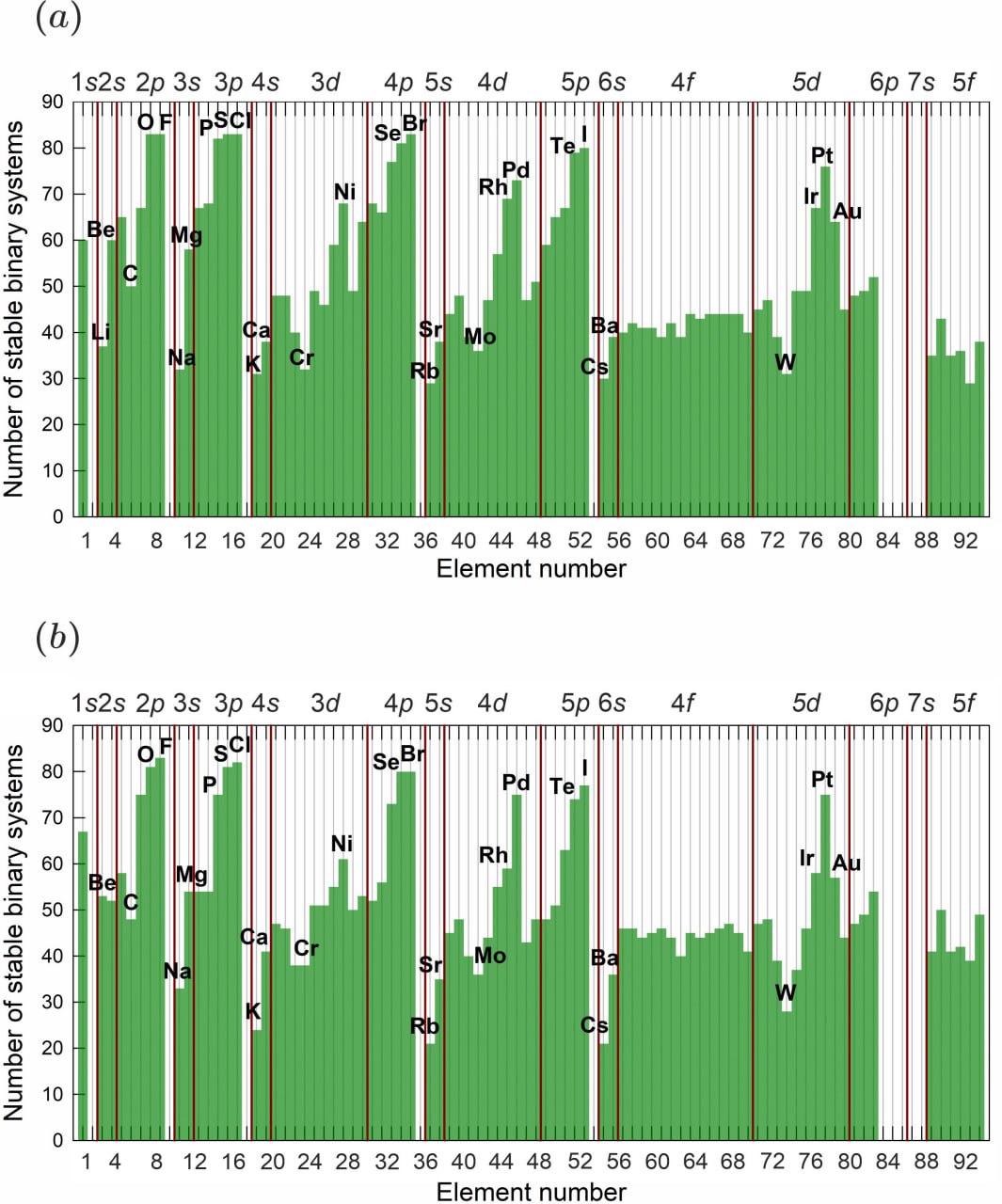
2. A Refined Electronegativity Scale: As part of this work, the team has derived an optimized X-scale from solid-state formation enthalpies. This scale demonstrates smoother periodicity and, in key cases like molybdenum, tungsten, and gold, corrects known overestimations present in Pauling's original scale, providing a more reliable metric for predicting bond polarity in solids.
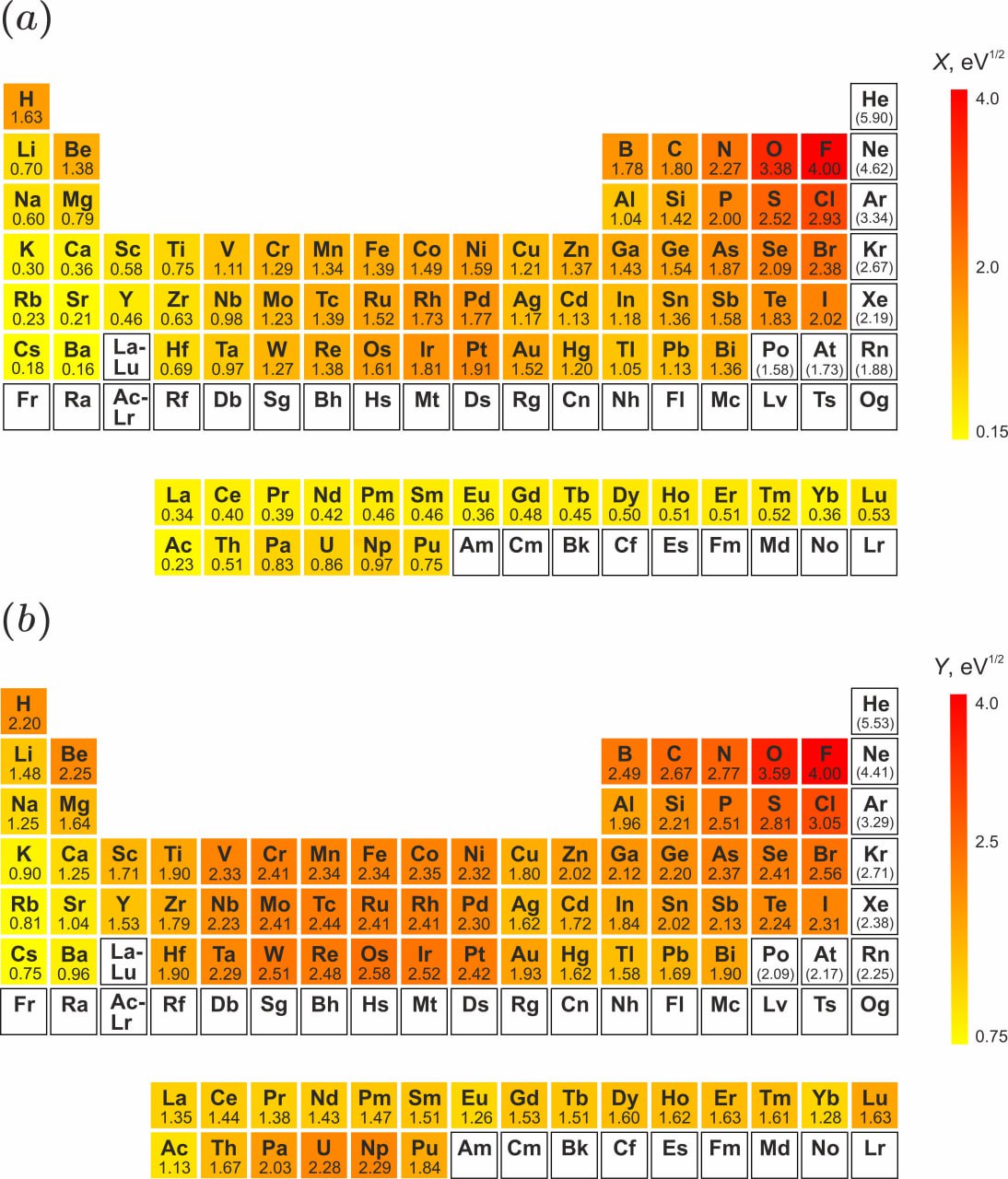
3. The Invention of "Stability Maps": A major innovation is the creation of compact stability maps for each element. These maps visually plot the calculated Q-value against every other element, providing an immediate, comprehensive overview of an element's chemical affinity. This novel representation is an invaluable tool for rapid insight.
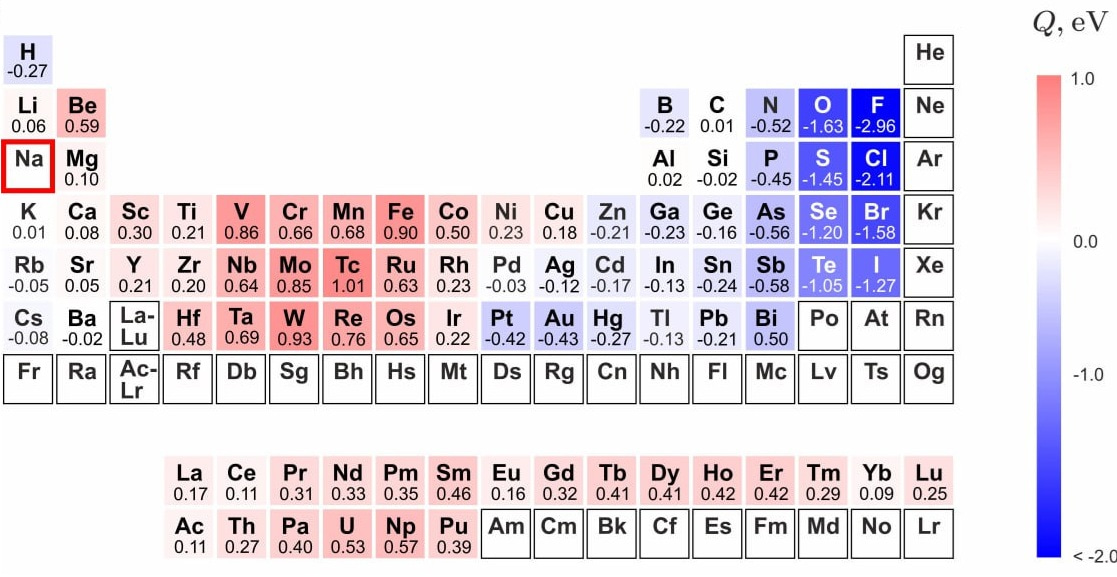
4. Direct Technological Application: This simple heuristic model directly assists in solving material challenges. For instance, the stability map for Sodium immediately identifies which metals (e.g., Fe, V, Mo, W) have the lowest chemical affinity to it, providing crucial guidance for selecting corrosion-resistant materials for advanced nuclear coolant systems.
5. From Fundamental Understanding to Design: Beyond binary systems, the framework explains the rich ternary chemistry of alkali metals (e.g., in carbonates and silicates), where the mismatch penalty is reduced. It also provides a basis for predicting high-pressure reactivity, where changes in electron density and electronegativity can induce new compound formation.
Conclusion: A Practical Bridge from Chemical Insight to Application
This work transforms a complex quantum-mechanical landscape into a simple, predictive model grounded in chemical physics. By quantifying the interplay between electronegativity and a mismatch factor, it explains non-obvious trends, introduces a refined electronegativity scale, and offers a new visualization paradigm through stability maps. The result is a powerful, practical tool that accelerates discovery by guiding researchers toward stable compounds and away from dead ends, with direct implications for technology and synthesis.
Link to the original paper: [Simple electronegativity-based model for predicting stable compound formation across the periodic table]
---
How might this model impact your field? Could stability maps change how you plan experiments or interpret chemical reactivity? Share your thoughts below.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in