Connecting Brain Organoids to Build Dynamic Neural Networks
Published in Bioengineering & Biotechnology, Neuroscience, and Biomedical Research
Introduction
The human brain contains billions of neurons organized into local circuits and long-range connections that link multiple distant regions, forming a complex network. Every thought, sensation, and memory emerges from the coordinated activity of these interconnected neurons and brain regions communicating through complex circuits. For decades, neuroscience has tried to understand these communication rules. How do local networks interact, how does patterns of activity arise, and how do they develop and change in disease.
Organoids are tiny, three-dimensional brain-like tissues grown from stem cells and they have revolutionized how we study brain development and disorders in a dish. These organoids exist as isolated aggregates of neurons and while they can mimic the formation of local networks, they are limited in modelling the long-range connections and tracts that link multiple brain regions together. Without these connections, their network activity remains limited, missing the higher-level interactions that are essential for brain functions. To address this limitation, we aimed to model such long-range projections that span multiple regions.

Building the Loop Connectoid
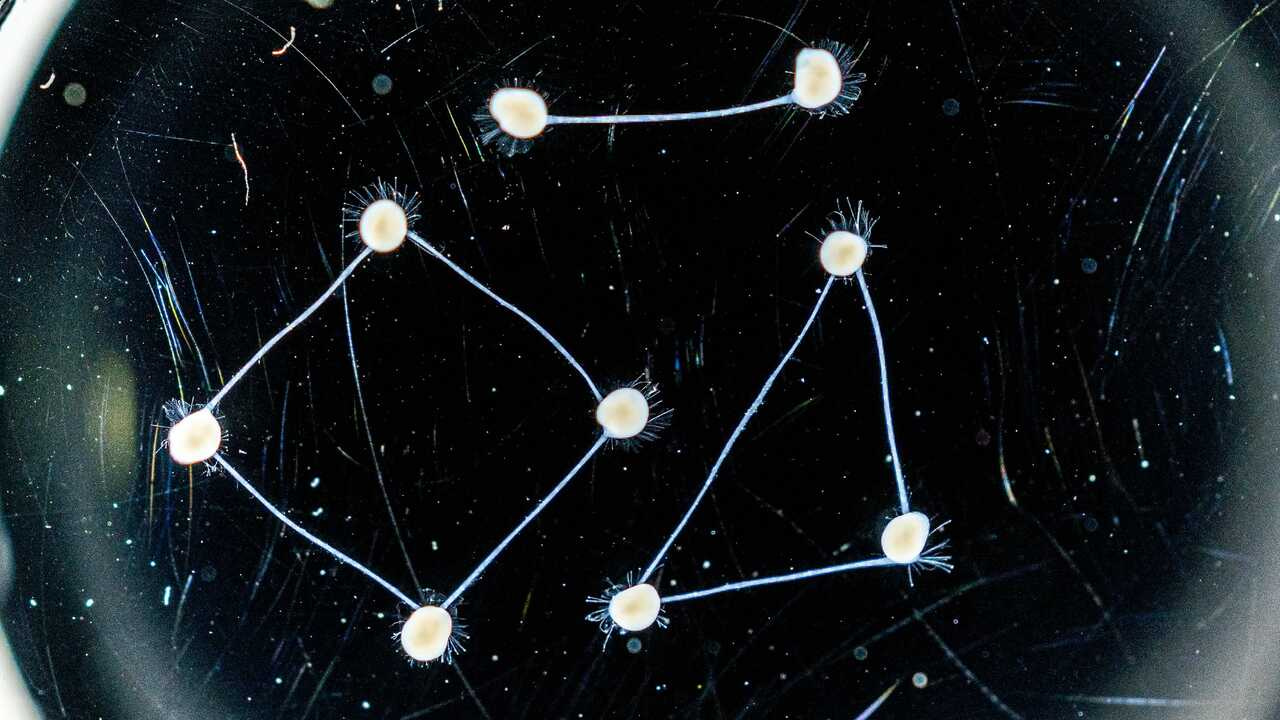
To make this possible, we developed a culture system that we call the loop connectoid. It consists of multiple brain organoids placed in small chambers that are linked by narrow microchannels inside a microfluidic device. The channels connecting the chambers guide axons growing out from the organoids to extend toward each other, forming bundles that act as bridges between them. This method is flexible and scalable, enabling a modular engineering approach, where organoids are used as building blocks to construct larger networks in a controlled manner. In this way, two, three or four organoids can be connected to form networks in specific configurations, such as in a loop structure. This approach not only enables controlled design of organoid networks but also adds a new structural feature to the organoid model, the axon bundle, allowing the investigation of how long-range connectivity shapes network function.

Multiple organoids can be connected with axon bundles that form connectoids
How Network Structure Drives Activity Dynamics
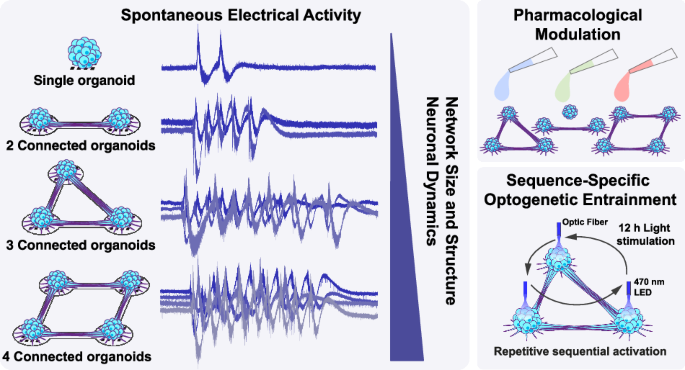
As we connected organoids and looked at the neural activity, we observed systematic changes in their patterns of activity. Single organoids exhibited spontaneous bursts of electrical activity, but once connected, these bursts became coordinated across the network. With additional connected organoids, the bursts grew richer, longer, and began to propagate through the network in wave like patterns. Each new organoid connection added feedback loops that enabled signals to circulate within the network. This recurrent communication prolonged bursts and increased the complexity of their timing. In this connected state, each organoid generated its own rhythms while responding to those of its neighbors, forming a self-sustaining network of interacting modules. The result was a more dynamic system suggesting that connecting organoids through axon bundles reshapes their network behavior and that functional complexity can emerge from a network-level design.
Activity propagation in a 4-membered loop connectoid
Structural Connectivity Enables Functional Modulation
Then came the most exciting part: we used optogenetics, a light-based method to stimulate each organoid in a defined sequence. After repeatedly activating them in a certain order for 12 hours, we found that the spontaneous activity of the network started to echo the same sequence more often, as if the circuit had adapted to it. This showed that this model is more than just a passive model, but that it can also exhibit adaptive behavior.

Sequential optogenetic stimulation of loop connectoids inside an incubator
This observation may offer insight into some principles that govern neural circuit formation. The connectoid system can provide the structural basis of connectivity, establishing a physical framework for communication and interactions. Once this structure is in place, their functionality can be further refined by external modulation, in our case, through optogenetic stimulation. In other words, the project demonstrates how some structural and functional components of neural circuits can be modelled and tuned step by step. First by engineering the connections, and then by guiding their activity.
Why it Matters
By building these organoid networks, we’re creating an experimental platform to tackle current limitations of the organoid model. This project aimed to reconstruct two essential features of brain organization: the structure and function of neural circuits. By creating the structural backbone of connectivity through axon bundles, and then refining activity patterns with optogenetic stimulation, we show how neural circuits can self-organize and adapt within a controllable, engineered framework.
Furthermore, the model represents a conceptual platform for studying how network architecture can shape function. Given the scalability and flexibility of the platform, we can now also design even larger networks or connect organoids representing different brain regions, such as cortical, hippocampal, or thalamic tissues. This could also allow us to reconstruct specific long-range circuits in a dish and explore how diseases like ALS or OCD disrupt communication across regions.
Looking into the Future
Beyond studying the connectoids in the lab, we are also exploring how living neural circuits might someday interact with the outside world. Through collaborations and art–science projects, we have created early prototypes that make this idea easier to imagine. With the DLX Design Lab, we have built “Talking with Neurons,” where people could speak into a microphone to trigger remote stimulation of organoids and see their responses turned into real-time sound and visuals. In a concept project called “Aura,” we imagined using cultured neural circuits to interpret chemical signals and explored the possibility of making circuits related to smell and decision-making. We have also worked with SoftBank Corp. and the artist Daito Manabe on the idea of a Brain Processing Unit (BPU), an organoid-based computing platform inspired by the adaptiveness and energy efficiency of living networks. These projects are not deployable systems yet, but they offer a practical glimpse of what future interaction with cultured neurons could look like. These activities have generated practical, testable research ideas, and they have directly contributed to the research of the loop connectoids.
Follow the Topic
-
Communications Biology

An open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of the biological sciences, representing significant advances and bringing new biological insight to a specialized area of research.
Your space to connect: The Psychedelics Hub
A new Communities’ space to connect, collaborate, and explore research on Psychotherapy, Clinical Psychology, and Neuroscience!
Continue reading announcementRelated Collections
With Collections, you can get published faster and increase your visibility.
Forces in Cell Biology
Publishing Model: Open Access
Deadline: Apr 30, 2026
Mechanistic insights into human host and microbiome interactions
Publishing Model: Open Access
Deadline: May 31, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in