A fungal pathogen drives the spread of a defensive symbiont in an insect host
Published in Ecology & Evolution
Pathogens face numerous challenges as they seek to invade and persist in hosts that can build defence by harbouring protective microorganisms. These ecological interactions indicate that pathogens act as crucial selective pressures that drive host microbe evolution. However, the evolutionary process of host microbes, or symbionts, in responding to changing selection pressures from pathogens, is still unknown. We set out to trace the pathogen-driven symbiont spread in nature by exploring correlations between an insect-protecting symbiont and a pathogen in various ecological niches.
Our research was conducted with the entomopathogenic fungus Cordyceps javanica and the whitefly Bemisia tabaci, which harbours symbionts including Rickettsia in natural niches across China from 2016 to 2021. Our field investigation revealed a significant increase in the frequency of whitefly covert infection by C. javanica over that period. Remarkably, symbiont Rickettsia frequencies also increased significantly in each population of whitefly, showing a positive correlation with the dynamics of C. javanica infection at regional, local and individual whitefly-infested plant scales. On the basis of these findings, a series of greenhouse and laboratory experiments were then conducted and confirmed that applying pathogen pressure could selectively drive Rickettsia to sudden fixation in whiteflies (that is, all whiteflies are infected with Rickettsia) both in the laboratory and in the field. Furthermore, the driving force is attributable to Rickettsia-conferred suppression of pathogen. Especially, no pathogen spores were released by Rickettsia-positive whitefly cadavers, guaranteeing the safe conditions for the development of offsprings before whitefly parents died. Consequently, with the competitive advantage of Rickettsia-positive whiteflies accumulated under the pathogen pressure, Rickettsia spread in the whitefly populations rapidly (Fig.1). Combining the results from the field investigations, greenhouse tests and lab experiments, we conclude that pathogens are an important driving force for rapid shifts of host symbionts in the natural niche.
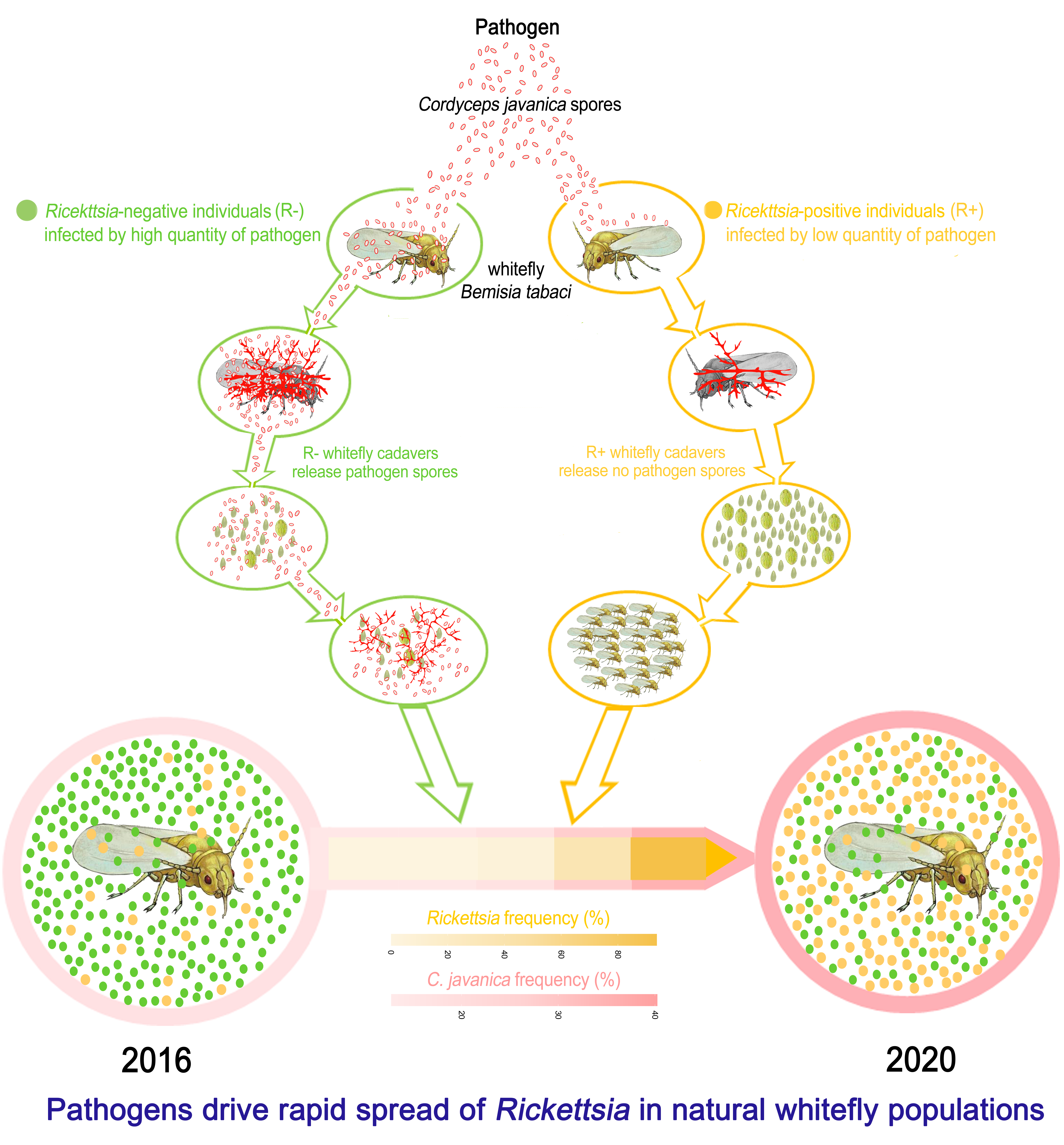
Fig.1 | Rapid spread, from 2016 to 2020, of the symbiont Rickettsia in its whitefly host is driven by the pathogen Cordyceps javanica. The driving force is elucidated by the Rickettsia-conferred suppression of pathogen infection quantity, proliferation and sporulation acting as a potential barrier of onward transmission of the pathogen.
During the last decade, we have been committed to developing new microbial pesticides and, at the same time, have been unravelling the coevolutionary relationships among the microbes and insects. This pathogen, Cordyceps javanica, was isolated from field whitefly cadavers in 2012; after 4 years of research to verify its feasibility, we began registering the pathogen as a microbial pesticide against whitefly in 2016. Meanwhile, we conducted experiments to investigate symbiont-conferred protection against the pathogen, and kept on monitoring the spread of both the pathogen and the symbiont in nature. In 2021, we successfully registered the pathogen as a pesticide and formed our theory that pathogens drive the rapid spread of the insect symbiont. Moreover, to assess whether C. javanica have potential for biocontrol of other agricultural pests, we have carried out lots of field and lab experiments. Intriguingly, C. javanica shows high levels of pathogenicity on rice planthoppers, aphids, and the two-spotted spider mite. Since a few months ago, our microbial pesticide made of C. javanica has been officially allowed to applicate for the biocontrol of the rice pest, brown planthopper Nilaparvata lugens. It is predictable that C. javanica, as a green option for sustainable pest management, has wide application prospect.

Fig.2 | The microbial pesticide we developed using the entomopathogenic fungus Cordyceps javanica spores and its application spectrum. A: The packages of the microbial pesticide product; B: C. javanica-infected whiteflies Bemicia tabaci on a tomato leaf; C: a C. javanica-infected brownplant hopper Nilaparvata lugens on a rice stem; D: a C. javanica-infected two-spotted spider mite Tetranychus urticae on a greenbean leaf.
In the future, we hope to decipher the mechanistic basis for symbiont-conferred protection, and trace how pathogens evolve in protected hosts. Despite it has been quite a struggle to develop practical techniques that can overcome symbiont-mediated protection against pathogens in pest biocontrol, fortunately, we have found ways to solve the problem — and we will tell this as our next story.
Follow the Topic
-
Nature Ecology & Evolution

This journal is interested in the full spectrum of ecological and evolutionary biology, encompassing approaches at the molecular, organismal, population, community and ecosystem levels, as well as relevant parts of the social sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Biodiversity and ecosystem functioning of global peatlands
Publishing Model: Hybrid
Deadline: Jul 27, 2026
Understanding species redistributions under global climate change
Publishing Model: Hybrid
Deadline: Jun 30, 2026






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in