A Kaposi’s sarcoma-associated herpes virus-encoded microRNA contributes to dilated cardiomyopathy
Published in Healthcare & Nursing
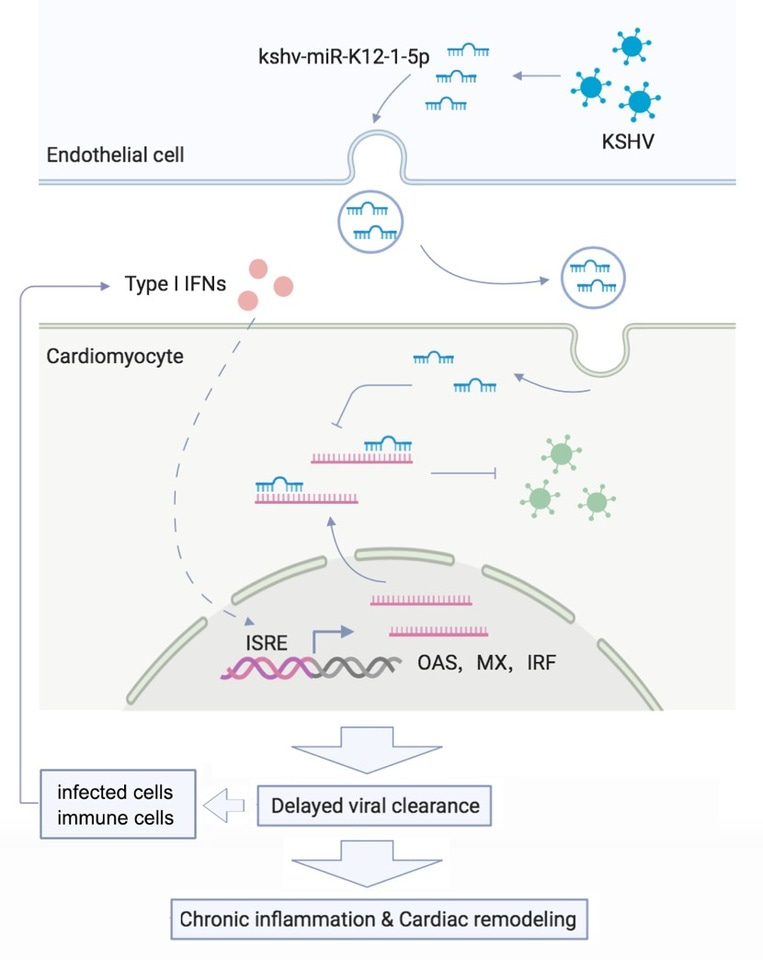
Dilated cardiomyopathy (DCM) is a heart muscle disease defined as left ventricular or biventricular dilation and systolic dysfunction in the absence of either pressure or volume overload or coronary artery disease that is sufficient to explain the dysfunction1. Although the outcome of DCM has improved in recent years because of earlier diagnosis and implementation of pharmacological and non-pharmacological therapeutic strategies, a considerable proportion of patients with DCM still have an unfavorable prognosis, particularly including the risk of substantial heart failure and arrhythmia2. Inflammation, activated by viral persistence, is a key trigger factor for cardiac remodeling and the development of DCM; this is called inflammatory cardiomyopathy3. Despite the well-known cardiotropic viruses, we found that a new form of virus, Kaposi’s sarcoma-associated herpes virus (KSHV), is a risk factor for DCM. We unraveled the mechanisms by which KSHV-encoded miRNA inhibits the type I interferon (IFN) signaling pathway, thereby aggravating known cardiotropic virus-induced cardiac dysfunction and inflammatory infiltration because of obstacles to virus clearance.
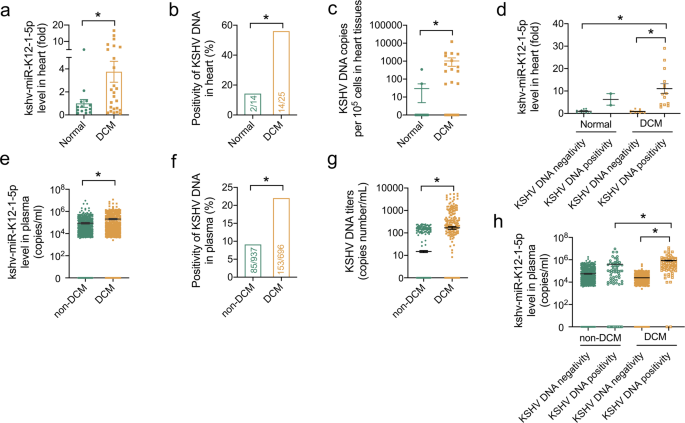
This study was based on the screening finding that viral miRNAs are increased in the heart and plasma samples from patients with DCM compared with controls. In particular, only one of these viral miRNAs, kshv-miR-K12-1-5p, was elevated in the heart and plasma of patients with DCM. During follow-up, we found a potential association between increased kshv-miR-K12-1-5p levels and KSHV prevalence with poor prognosis of DCM, exciting our interest in the role of kshv-miR-K12-1-5p in DCM.
We then investigated the possible cellular origin of cardiac kshv-miR-K12-1-5p and detected it in endothelial cells and cardiomyocytes, whereas KSHV was exclusively detectable in endothelial cells in KSHV-positive DCM heart samples. Using exosome analysis and coculture, we observed the transfer of kshv-miR-K12-1-5p from KSHV-infected endothelial cells to cardiomyocytes.
At the beginning of our investigation of these mechanisms, we wondered whether KSHV infection could impair cardiac function. However, we failed to infect the animal model with KSHV because its natural host is limited to humans4. We focused on the function of kshv-miR-K12-1-5p and overexpressed it in vitro using recombinant adeno-associated virus (rAAV). Overexpression of kshv-miR-K12-1-5p did not alter cardiac function under normal conditions or in a pressure overload model induced by transverse aortic constriction. As inflammation is a key trigger factor in DCM and DCM hearts with KSHV DNA positivity seem more susceptible to other cardiotropic viruses, we explored the role of kshv-miR-K12-1-5p in virus-induced cardiac inflammation. We found that kshv-miR-K12-1-5p aggravated cardiotropic virus-induced cardiac inflammatory infiltration and dysfunction and the release of inflammatory cytokines.
This study also investigated the molecular function of kshv-miR-K12-1-5p via Argonaute2 RNA immunoprecipitation sequencing, biotin-labeled miRNA pull-down assay, luciferase reporter assays, and western blotting. We found that kshv-miR-K12-1-5p disrupts the type I IFN signaling pathway by directly targeting its downstream genes. In addition, kshv-miR-K12-1-5p did not affect inflammatory cell infiltration or cardiac function after type I IFN signaling was blocked using an anti-IFNAR neutralizing monoclonal antibody. These data suggest that kshv-miR-K12-1-5p acts on the IFN signaling pathway to enhance the virus-induced cardiac inflammatory response.
In conclusion, this study provides insight into DCM involving a virus and its miRNA. We found that human cardiac endothelial cells infected with KSHV release kshv-miR-K12-1-5p, which disrupts the type I IFN signaling pathway in cardiomyocytes. Thus, KSHV can increase subsequent infections by other known cardiotropic viruses. Delayed viral clearance contributes to chronic inflammation, cardiac remodeling, DCM development, and the production of type I IFNs via microenvironment feedback mechanisms (Figure 1).
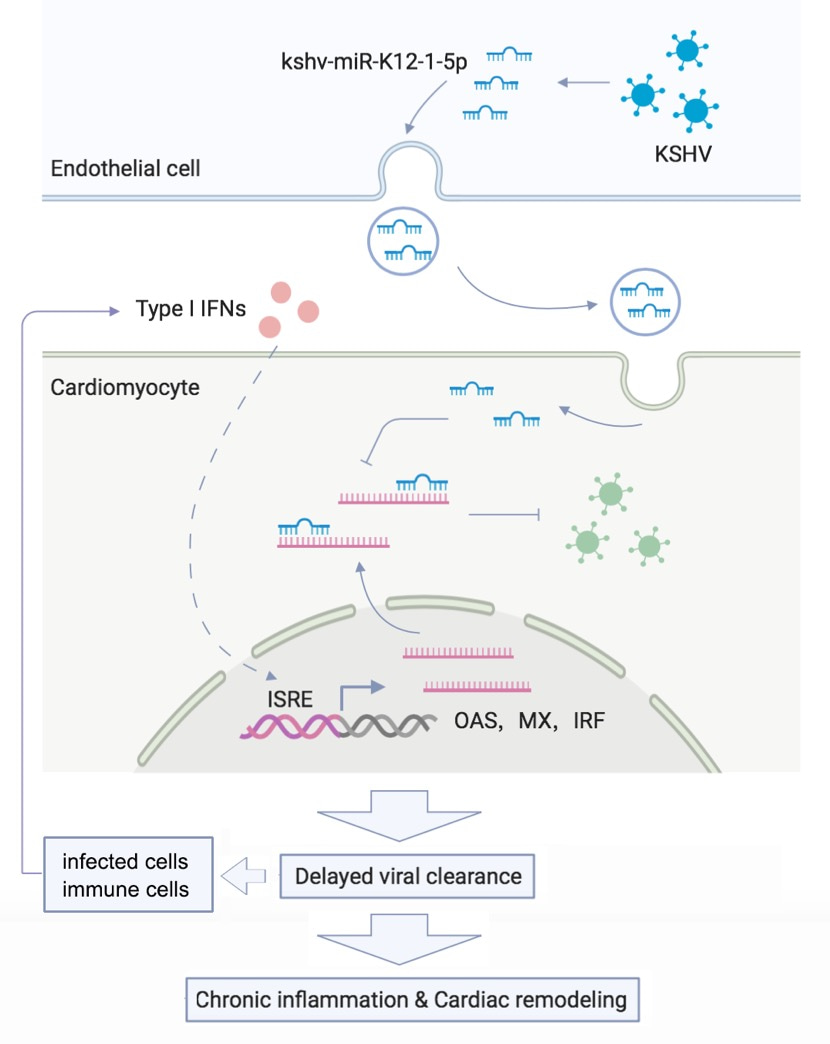
Figure 1. A Kaposi’s sarcoma-associated herpes virus-encoded microRNA contributes to dilated cardiomyopathy.
For more detail on the experiments and results, please read our paper https://www.nature.com/articles/s41392-023-01434-3.
References
1 Sinagra, G., Elliott, P. M. & Merlo, M. Dilated cardiomyopathy: so many cardiomyopathies! Eur. Heart J. (2019).
2 Heidenreich, P. A. et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 145, e876-e894 (2022).
3 Schultheiss, H. P. et al. Dilated cardiomyopathy. Nat. Rev. Dis. Primers 5, 32 (2019).
4 Naipauer, J. & Mesri, E. A. The Kaposi's sarcoma progenitor enigma: KSHV-induced MEndT-EndMT axis. Trends Mol. Med. 29, 188-200 (2023).
Follow the Topic
-
Signal Transduction and Targeted Therapy

This is an international, peer-reviewed, open-access journal publishing articles related to signal transduction in physiological and pathological processes, alongside signal transduction-targeted therapeutics in the form of biological agents and small molecular drugs used to treat human diseases.
Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in