A Roadmap from the Bond Strength to the Grain-Boundary Energies and Macro Strength of Metals
Published in Materials

It has been a long-term goal to directly correlate bond strength with the strength of metals, aiming to access the upper bound of strength. With the growing demand for high-performance materials, increasingly complex alloys are being studied and applied, such as the multi-principal-element alloys and amorphous states. However, the structure-property relationships between bond strength and macro-mechanical properties across different metallic systems pose significant challenges due to the vast composition spaces and complex structures of metals, particularly MPEAs. These obstacles necessitate the development of simple and effective physics-based descriptors to facilitate the material design.
On the basis of the approximate linear correlation between GB energies and surface energies, we now propose two expressions to determine the GB energies in the view of the tight-bonding theories. One is,
EGB∝γsurf∝EcohCNGB∝Ecoh/r05
for the strong bond metals with a minor bond relaxation according to the broken-bond model. The other is,
EGB∝γsurf∝EcohCNGB1/2∝Ecoh/r02.5
for the relatively weak bond metals with a significant bond relaxation according to the square-root broken-bond model. These two descriptors are able to accurately determine the GB energies regardless of the BCC, FCC and HCP metals as well as the various GB types and orientations. Our results uncover the different behavior of GB stability for TMs and MGMs: Ecoh/r05 applies to the metals with strong bonds and large cohesive energies (mainly TMs) while Ecoh/r02.5 is applicable to those with weak bonds and small cohesive energies (mainly MGMs).
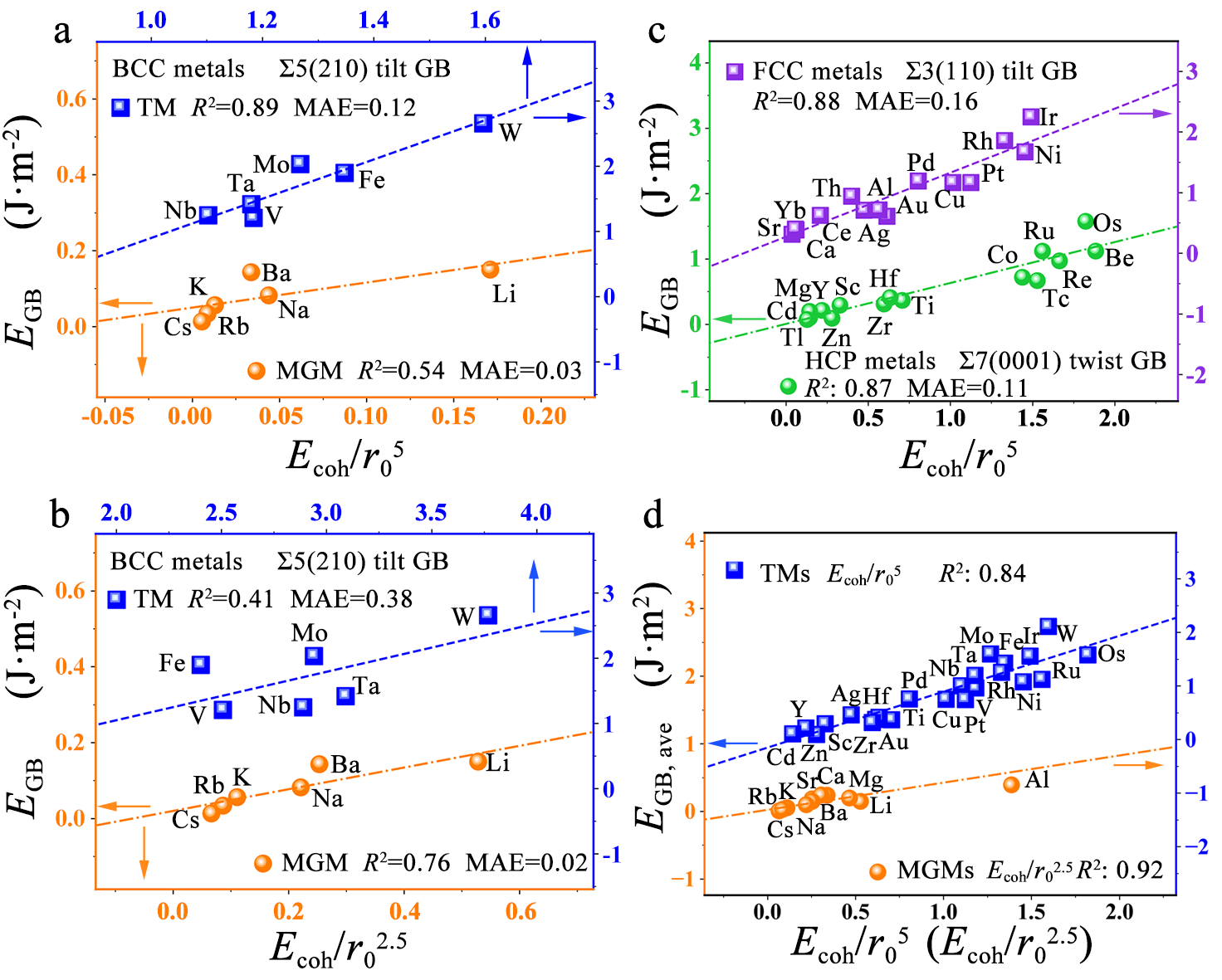
Figure 1. Grain-boundary (GB) energies (EGB) as the functions of our descriptors. GB energies with (a) Ecoh/r05 and (b) Ecoh/r02.5 for body-centered-cubic (BCC) metals. GB energies with (c) Ecoh/r05 for hexagonal-close-packed (HCP) and face-centered-cubic (FCC) metals. (d) The average GB energies with Ecoh/r05 for TMs and Ecoh/r02.5 for MGMs.
The proposed bond-strength descriptors based on the classical tight-binding theories are also strongly correlated with the interstitial electron density ρ0 and the heat of fusion kBTm/Ω0, indicating solid physical origin of the proposed descriptors. Meanwhile, our descriptors can naturally be used to quantify the bond-strength-related macro strength of metals, such as the bulk modulus and the ultimate strength.
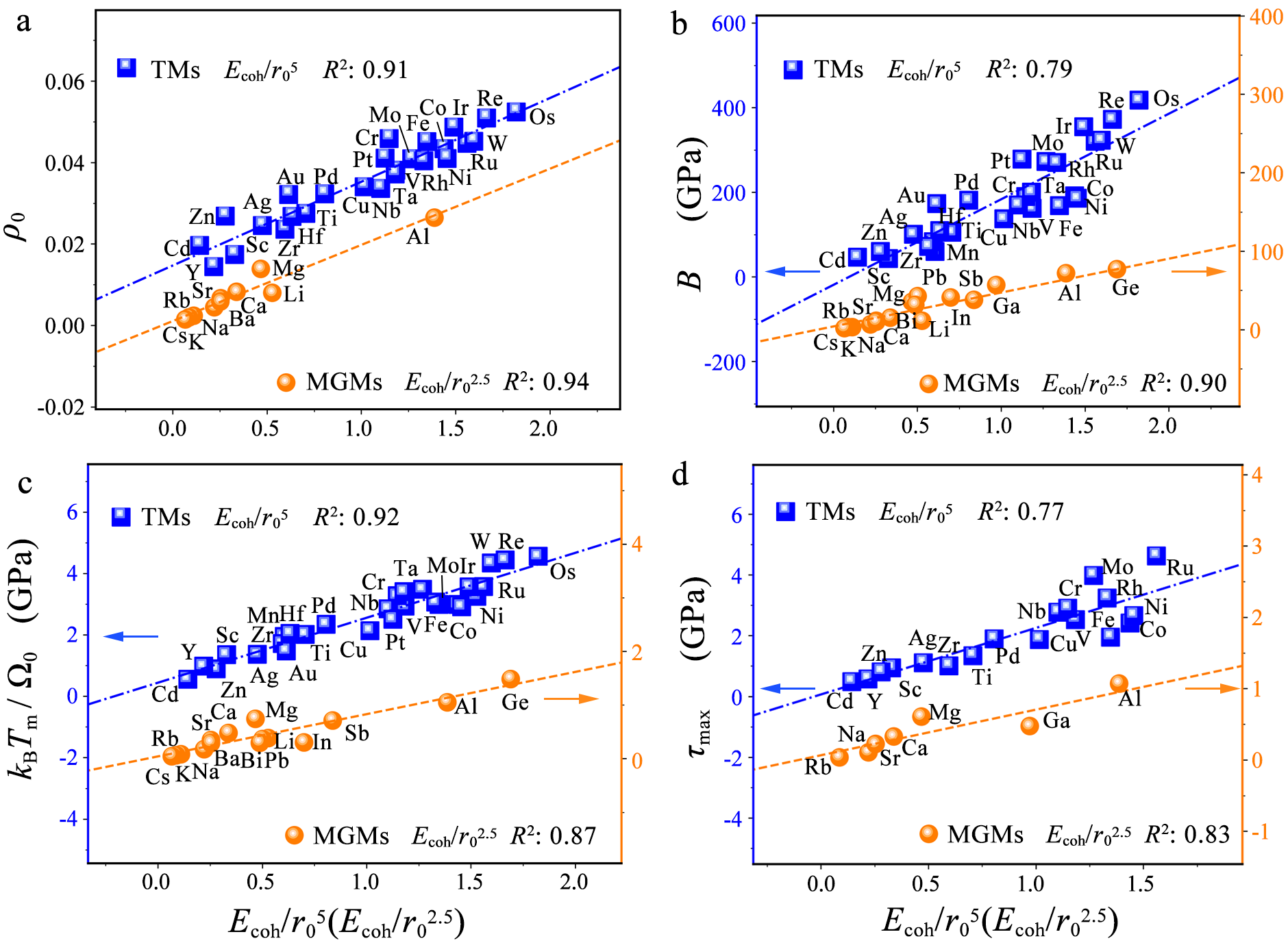
Figure 2 The correlation between our descriptors, the previously proposed characteristics and the macro properties of different transition metals (TMs) and main-group metals (MGMs). (a) The interstitial electron density ρ0. (b) The bulk moduli B. (c) The average heat of fusion kBTm/Ω0. (d) The maximum shear strength τmax.
By combining with the rule-of-mixture (RoM) estimate, our descriptors can be extended to determine the macro strength of high-entropy alloys (HEAs). We find that (Ecoh/r05)RoM, instead of(Ecoh/r02.5)RoM, exhibits a linear correlation with Young’s modulus for Al-Co-Cr-Cu-Fe-Mn-Ni based 3d HEAs and refractory-metal-based HEAs, indicating Young’s modulus of the HEAs composed of metals with the strong bond ability are mostly affected by the repulsive effects of bonding.
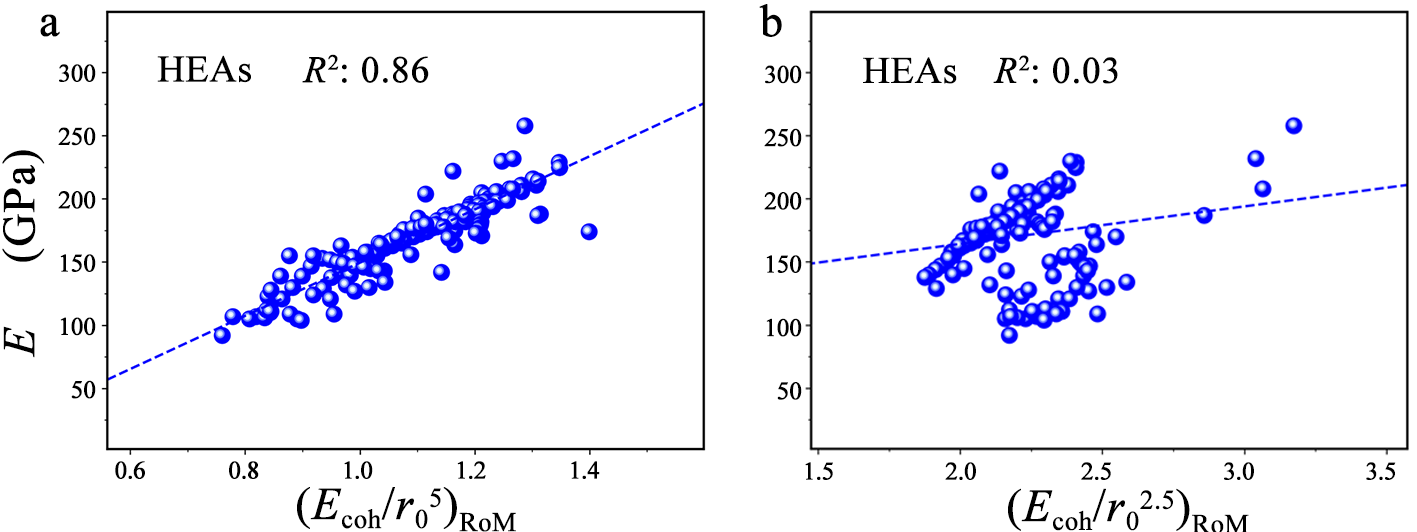
Figure 3 Macro properties of high-entropy alloys (HEAs) as the functions of our descriptors. Young’s moduli E with (a) (Ecoh/r05)RoM and (b) (Ecoh/r02.5)RoM for Al-Co-Cr-Cu-Fe-Mn-Ni-based 3d HEAs and refractory-metal-based HEAs.
Interestingly, we find that our framework is also suitable in determining the strength properties of bulk metallic glasses (BMGs), although BMGs have no lattice structures and GBs compared to HEAs. (Ecoh/r02.5)RoM, rather than (Ecoh/r05)RoM, is effective in determining the measured modulus E, fracture strength σf and glass-transition temperature Tg of BMGs. These results uncover the fact that the significant bond relaxation of BMGs enlarges the interstitial atomic space and weakens the bond strength, making TM and MGM elements behave consistently in the amorphous states with a law of (Ecoh/r02.5)RoM.
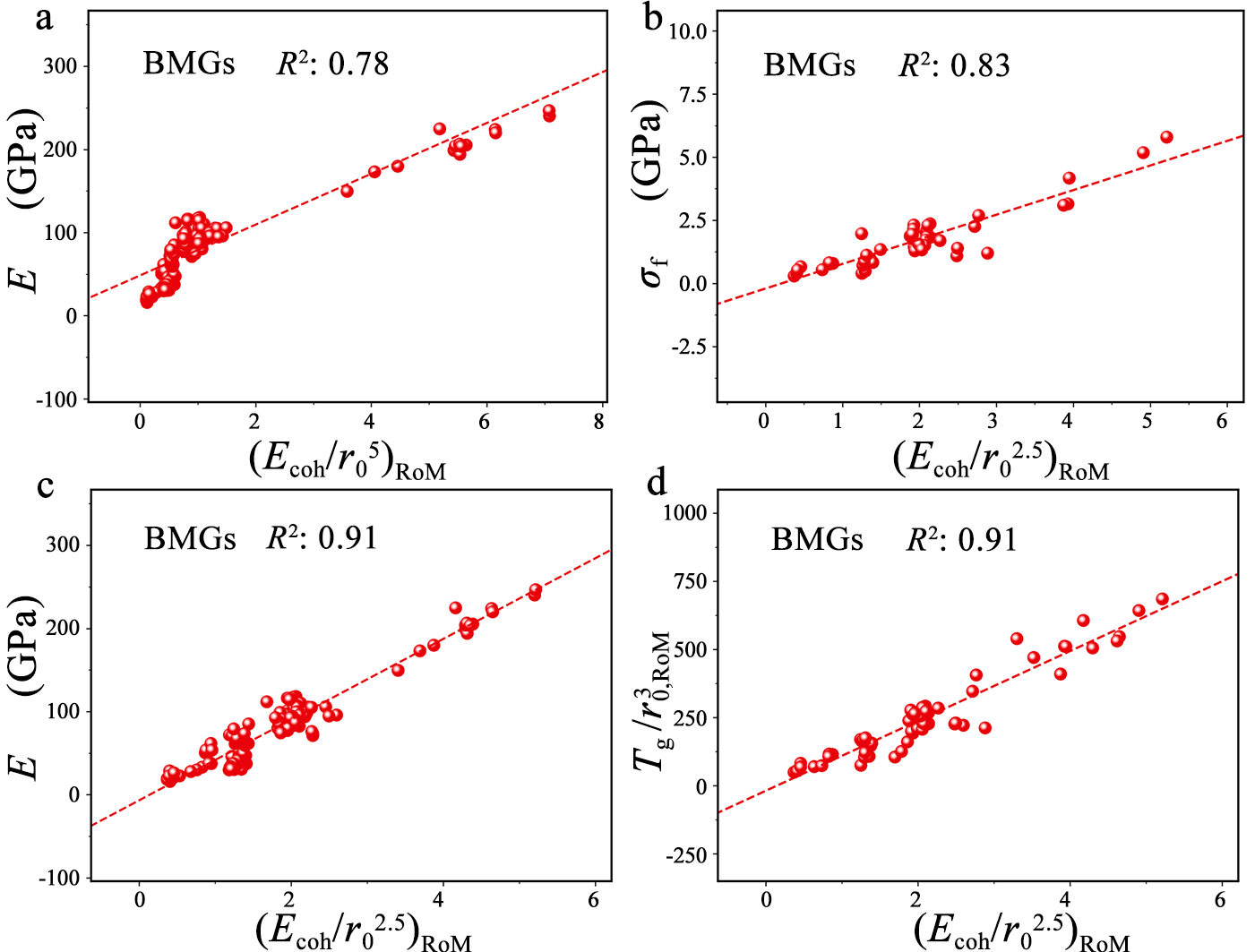
Figure 4 Macro properties of bulk metallic glasses (BMGs) as the functions of our descriptors. Young’s moduli E with (a) (Ecoh/r05)RoM and (b) (Ecoh/r02.5)RoM. (c) Fracture strength σf with (Ecoh/r02.5)RoM, and (d) the descriptor Tg/ with (Ecoh/r02.5)RoM for for Zr-, Cu-, Ti-, Mg-, Fe-, and alkaline-earth-based BMGs (in the form of binary and multiple-elemental alloys).
In summary, we propose the descriptors based on the cohesive energy and atomic radius to characterize the bond strength of metals, and establish the quantitative structure-property relationship from bond strength, to GB energies, and to macro strength. Our descriptors, deriving from the d-band characteristics and broken-bond mechanism of tight-binding models, are applicable to not only the pure metals (including TMs and MGMs), but also the HEAs and BMGs, showing good prediction accuracy and wide applicability with reference to the experimental and theoretical results. This framework reveals physical pictures that the repulsive (attractive) effects play the kernel role in the variation of the bond-strength related properties for TMs and HEAs (MGMs and BMGs), which thus elucidates the role of elemental compositions, lattice structures, high-entropy effects, and amorphous effects in determining the strength of metals. Our scheme also reflects the heat of fusion, interstitial electron density, and inscribed sphere radius of the electron gas, exhibiting a robust physical basis. These findings not only serve as the solid physical guidance for understanding the relationship between bond strength and macro properties from the atomic-level perspective, but also provide an effective tool (also applicable to combine with machine-learning approaches) for accelerating the design of alloys with high performance, especially for MPEAs.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in