A tale of two fusion proteins: RSV-F and hMPV-F
Published in Bioengineering & Biotechnology and Biomedical Research
Respiratory syncytial virus (RSV) and human metapneumovirus (hMPV) are two closely related viruses that cause respiratory infections in humans. Although they usually cause mild cold-like symptoms in healthy adults, severe infections can result in hospitalization or even death in infants, the elderly, or immunocompromised individuals. Three vaccines have recently been approved for RSV, but their protection wanes over time. As of yet, there is no vaccine against hMPV. Our ultimate goal is to create a bivalent vaccine that will provide long-lasting protection against both RSV and hMPV using our self-assembling protein nanoparticle (SApNP) platform. We have previously used SApNPs to display fusion proteins from other viruses, including HIV, filoviruses, and SARS-CoV-1 and -2, and shown that SApNPs elicit a stronger, longer-lasting immune response than the much smaller individual proteins. In this paper, we start by using structural analysis to design improved vaccine antigens for RSV and hMPV.
RSV and hMPV fusion proteins
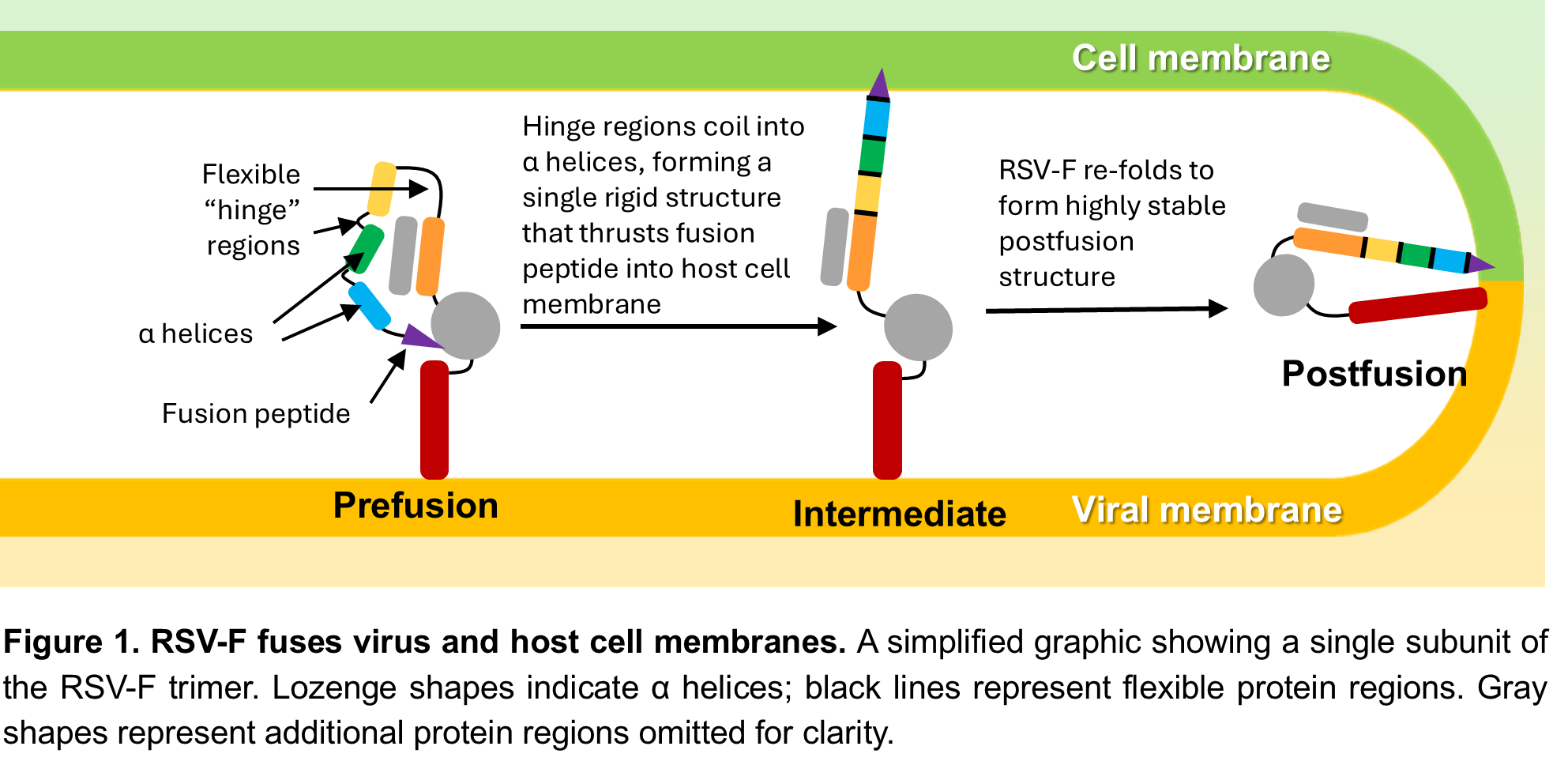
Like many viruses, RSV and hMPV enter host cells using fusion proteins (RSV-F and hMPV-F, respectively), which stud the viral surface in sets of three called trimers. RSV-F and hMPV-F use a similar mechanism to fuse viral and host cell membranes – in Figure 1 we use RSV-F as an example. In its prefusion form, the RSV-F proteins are folded over so that the fusion peptides – the part that gets inserted into the host cell membrane – are hidden within the closed trimer. When the trimer opens, the proteins quickly unfold into a straight, rigid structure that thrusts the fusion peptide into the host cell membrane. Finally, the proteins refold into the postfusion structure, pulling the viral and host cell membranes together (Figure 1). This process works because RSV-F is metastable: the prefusion form is much less stable than the postfusion form, but slightly more stable than the intermediate form it must go through to get there. Consequently, prefusion RSV-F is constantly teetering on the edge of shifting into the postfusion conformation – it just needs a “nudge” to get it started. Some fusion proteins need a specific signal, like binding to a host cell receptor or a change in pH, to change to the postfusion form, but RSV-F seems to flip from prefusion to postfusion form spontaneously. This is an issue for vaccine development because neutralizing antibodies (NAbs) need to bind to the prefusion form to prevent infection. To effectively stabilize RSV-F in the prefusion form, we needed to better understand why it so easily flips from prefusion to postfusion. Once we understood the basis of RSV-F’s metastability, we hoped to apply a similar strategy to hMPV-F, which has a very similar structure.
Existing prefusion-stabilized RSV-F designs
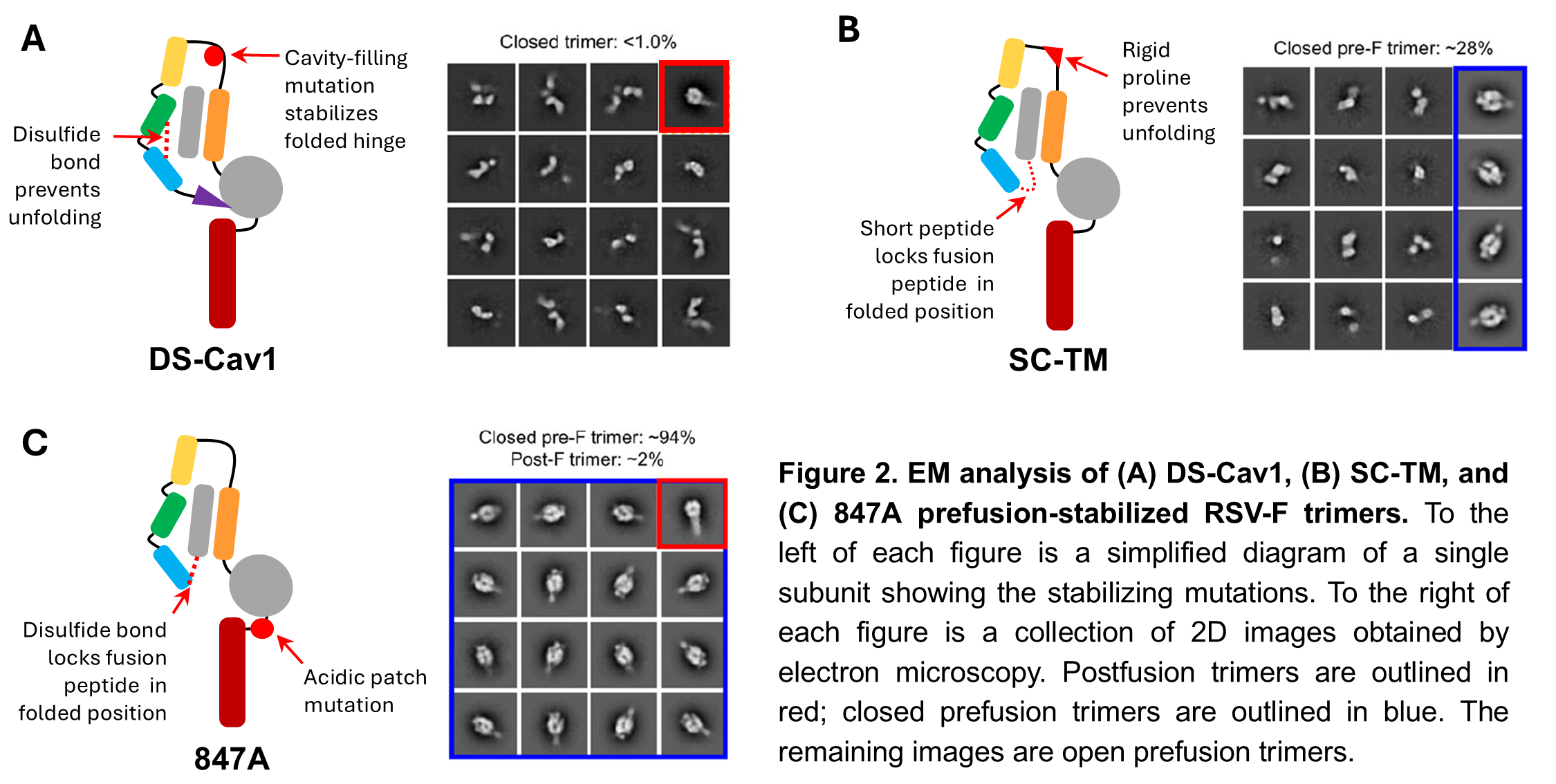
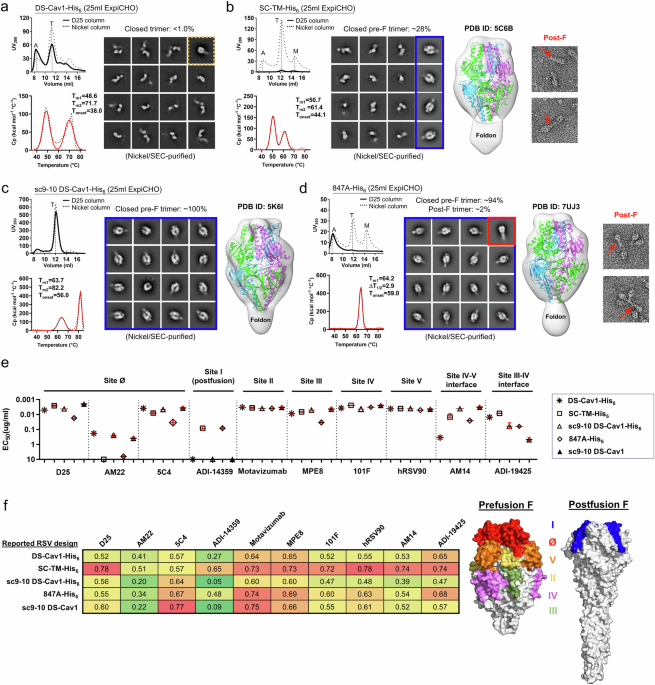
First, we used electron microscopy (EM) to analyze existing prefusion-stabilized RSV-F designs, several of which are used in currently licensed vaccines. Two designs, DS-Cav1 and SC-TM, effectively locked the individual RSV-F proteins in the prefusion form, but didn’t hold the trimer closed (Figure 2A-B). This is important because some NAbs specifically recognize the closed trimer. Another design, sc9-10 DS-Cav1, was similar to DS-Cav1 but included additional chemical bonds between the individual protein subunits to hold the trimer closed. This design gave 100% closed prefusion trimers, but we wanted to avoid these types of bonds in our vaccine construct because they would be incompatible with our SApNP platform, which is an important part of our ultimate vaccine strategy. Finally, the 847A design used a strong chemical bond to lock the fusion peptide in the folded position similar to DS-Cav1 and SC-TM, but also included a unique mutation in an area near the base of the trimer called the “acidic patch.” This design generated about 94% closed, prefusion trimers (Figure 2C). Clearly, the acidic patch mutation was important, but why? We found that certain amino acids within the acidic patch repelled each other, like a spring constantly trying to force the trimer open. We hypothesized that if we disabled this “spring,” we could create stable prefusion RSV-F trimers that would stay closed without the need for chemical bonds between the individual RSV-F subunits.
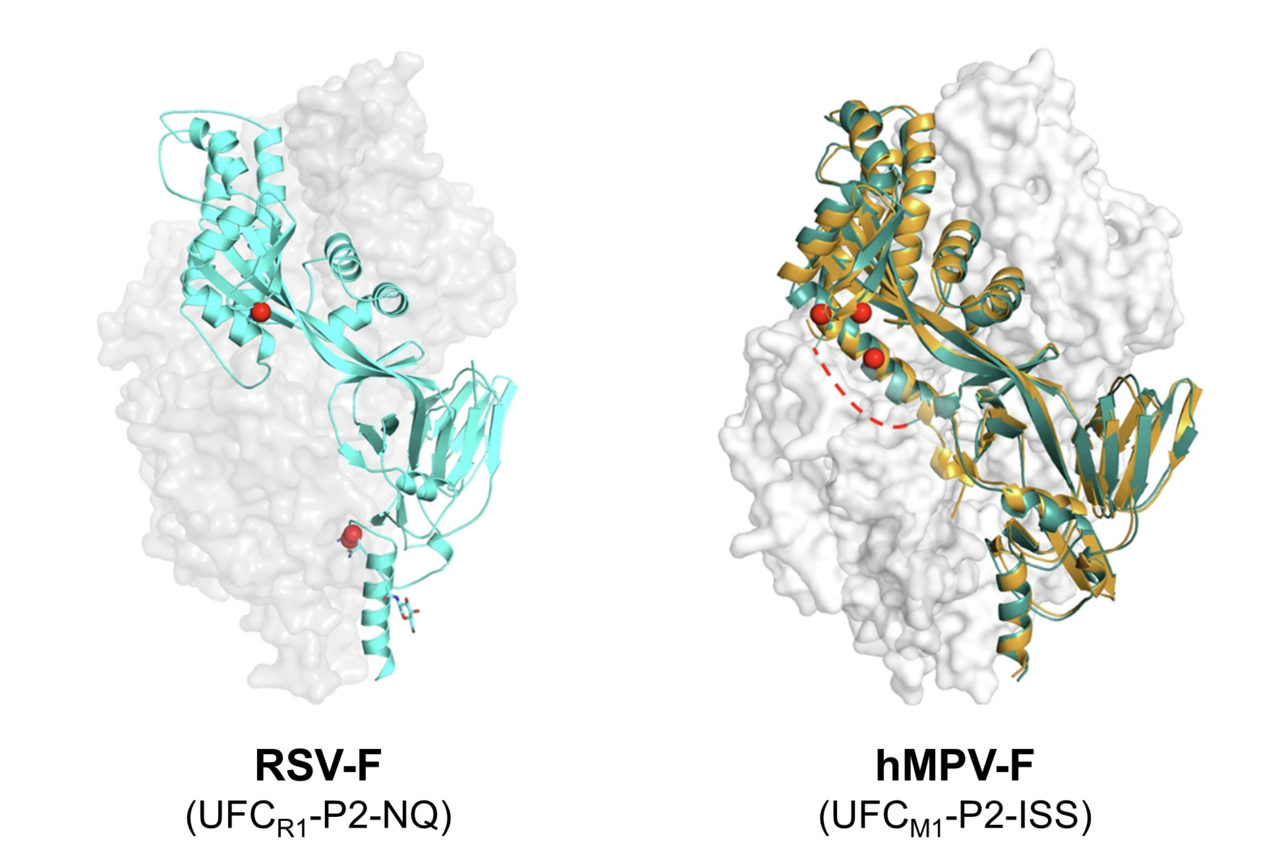
Our uncleaved prefusion-closed (UFC) RSV-F design
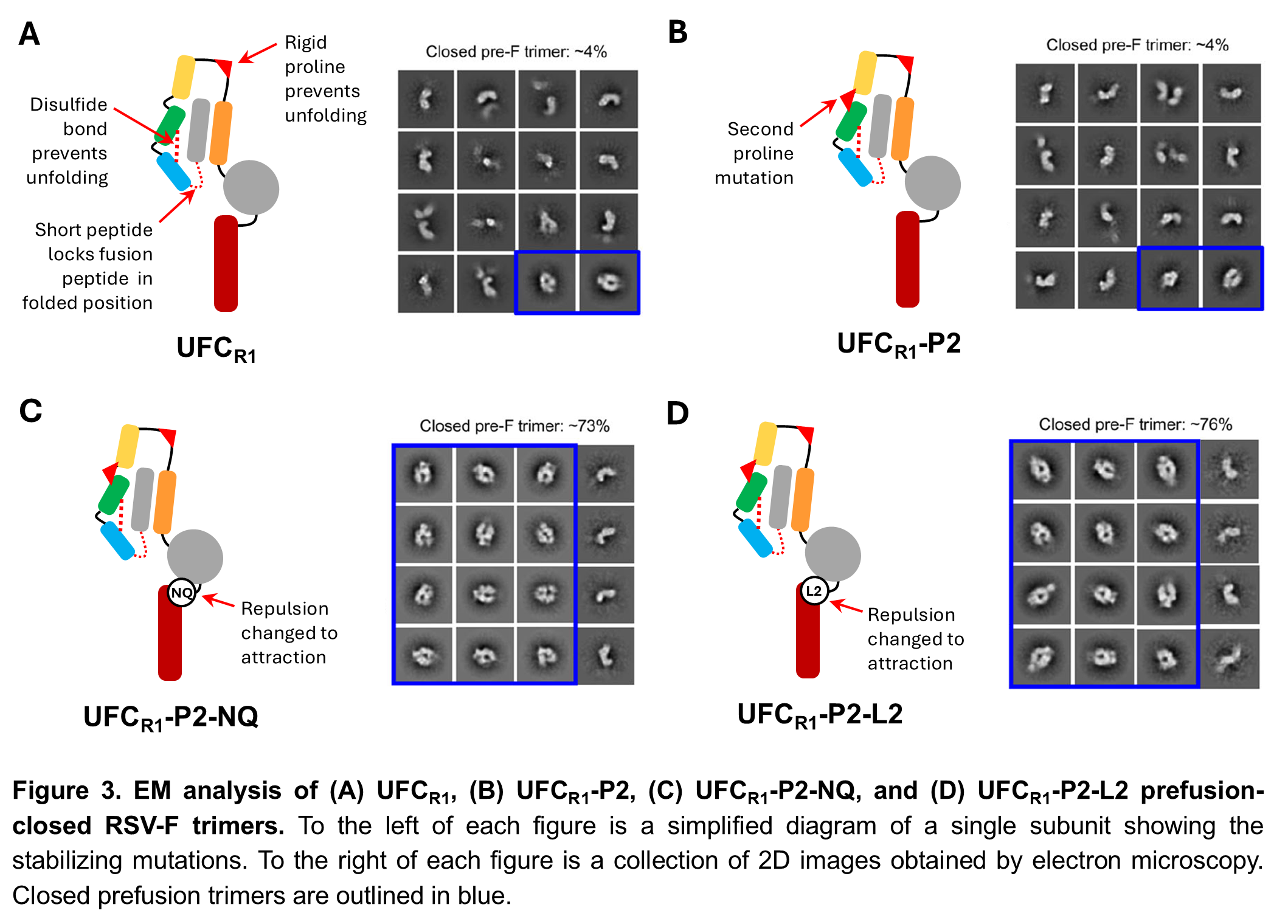
To test our hypothesis, we created four uncleaved, prefusion-closed (UFC) RSV-F designs. The first design, UFCR1, combined the most effective prefusion-stabilizing mutations from DS-Cav1 and SC-TM (Figure 3A). The second design, UFCR1-P2, added a second proline mutation to further stabilize RSV-F in the folded, prefusion conformation (Figure 3B). Both of these designs gave a much higher yield of prefusion RSV-F trimers than the existing designs we’d already tested, but they were still mostly open trimers. We then created two more designs, UFCR1-P2-NQ and UFCR1-P2-L2, with the same mutations as UFCR1-P2 plus mutations in the acidic patch designed to change the repulsive force between RSV-F subunits into an attractive one (Figure 3C-D). As we’d hoped, this significantly improved the proportion of closed prefusion trimers, from about 4% for the first 2 designs to over 70%.
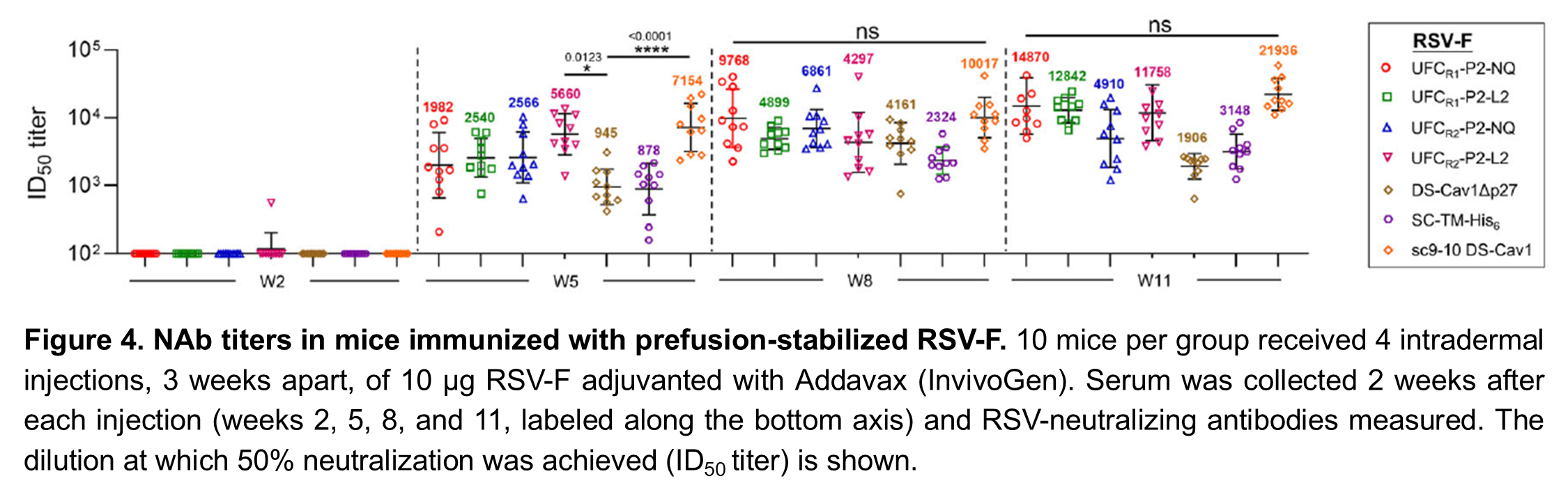
Finally, we tested our UFCR1 designs, as well as the existing ones, in mice. The results confirmed that closed prefusion trimers are important for an effective vaccine: of the existing designs, DS-Cav1 and SC-TM elicited the lowest levels of NAbs in mice and sc9-10 DS-Cav-1, which had 100% closed prefusion trimers, had the highest NAb levels. Our UFCR1-P2-NQ and UFCR1-P2-L2 designs elicited NAb levels similar to sc9-10 DS-Cav1, proving that its possible to produce an effective prefusion-stabilized RSV-F vaccine by eliminating the repulsion “spring” that drives trimer opening instead of using chemical bonds to force the trimer closed (Figure 4).
Our UFC hMPV-F design
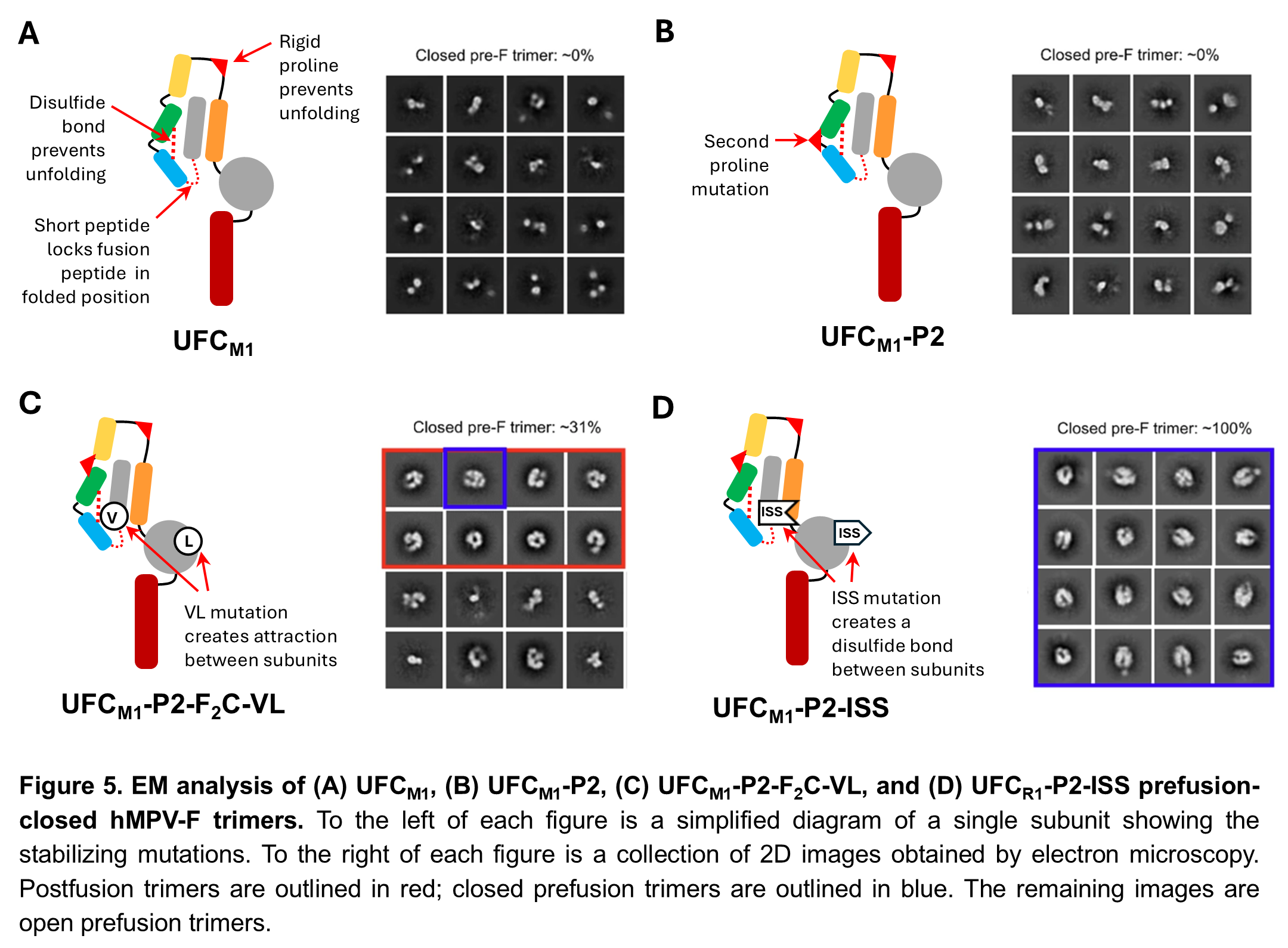
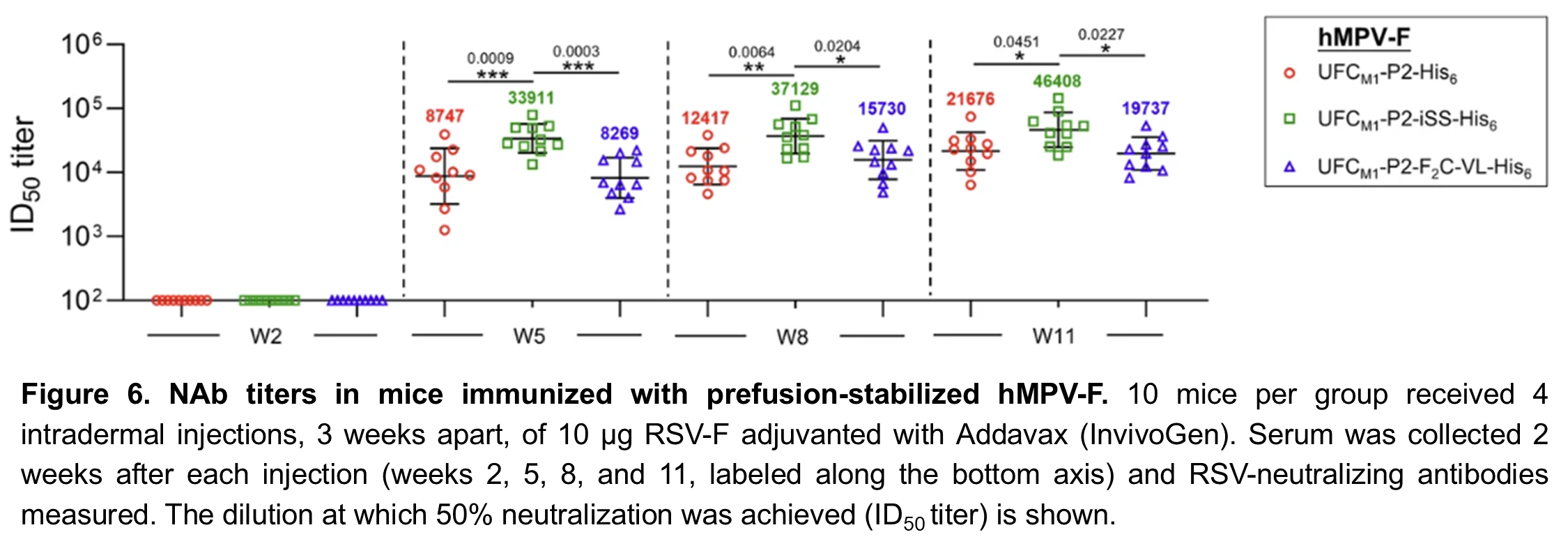
Following the success of this minimalist approach for RSV-F, we performed a similar structural analysis on hMPV-F. Our first two UFC hMPV-F designs, UFCM1 and UFCM1-P2, were similar to UFCR1 and UFCR1-P2, respectively, and like them produced almost entirely open trimers (Figure 5A-B). Despite their structural similarity, we found that hMPV-F doesn’t have a repulsive acidic patch like RSV-F, so we had to use a different strategy to produce closed trimers. Our third design, UFCM1-P2-F2C-VL, included a pair of mutations to create an attractive force between subunits. This was an improvement, but still only gave about 31% prefusion closed trimers (Figure 5C). In our 4th and final design, UFCM1-P2-ISS, we used disulfide bonds between subunits to obtain 100% closed prefusion trimers (Figure 5D). Although we want to avoid these types of bonds in our final vaccine, this gave us an important starting point for our future research. When we tested these hMPV-F designs in mice, we found that the UFCM1-P2-ISS design elicited higher NAb levels than UFCM1-P2 or UFCM1-P2-F2C-VL, confirming that closed trimers are important for an effective vaccine (Figure 6).
In our future work, we will continue to improve our UFC hMPV-F design to create prefusion closed trimers that can be displayed on our SApNP platform. We will then create SApNPs displaying multiple copies of UFC RSV-F and hMPV trimers. By combining these SApNPs with an effective adjuvant, we hope to create a bivalent vaccine that will provide effective, long-lasting protection against both RSV and hMPV.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026
Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in