An intranasally administered chimeric protein antigen, rCTB-T2544, orchestrates the generation of extended milieu of protective antibodies and T cell responses in the intestine, fortifying anti-typhoidal immunity in mice.
Published in Bioengineering & Biotechnology and Immunology
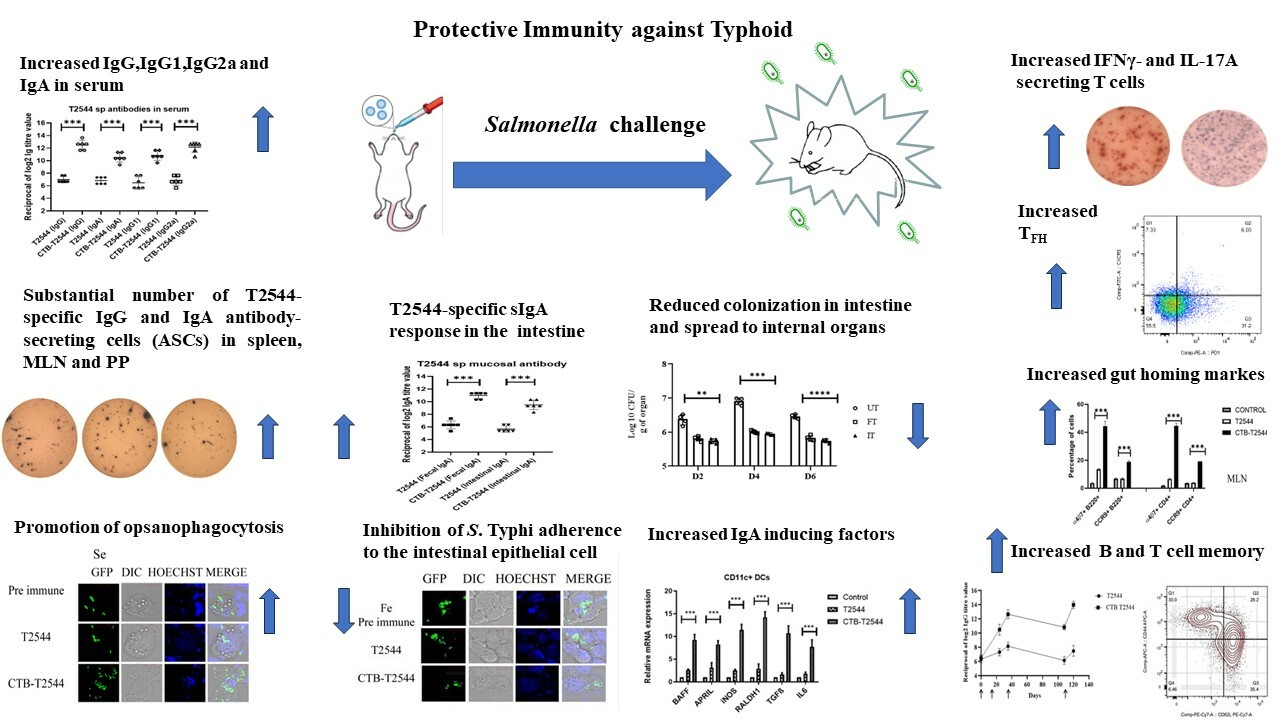
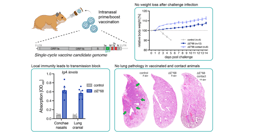
Backdrop- Development of safe, highly effective and affordable enteric fever vaccines is a global health priority. Commercialized Typhoid vaccines are modestly effective and work through the induction of systemic antibody response, whereas efficacy of licensed oral vaccine is compromised due to oral tolerance. Considering the entry of the causative agent, Salmonella Typhi through the feco-oral route, mucosal immune response in the intestine is highly desirable. To achieve this, our recently published article1 reported an immunogenic formulation, called rCTB-T2544, which contained the recombinant fusion protein of the B subunit of cholera toxin (CTB) from Vibrio cholerae and Salmonella Typhi outer membrane protein, T2544. We encourage a detail read into our work, where we showed that a candidate vaccine, delivered through the nasal route effectively induced intestinal secretory IgA (sIgA) and serum IgG, in addition to T cell memory response, leading to protection against the lethal challenge of S. Typhi in an iron-overload mouse model.
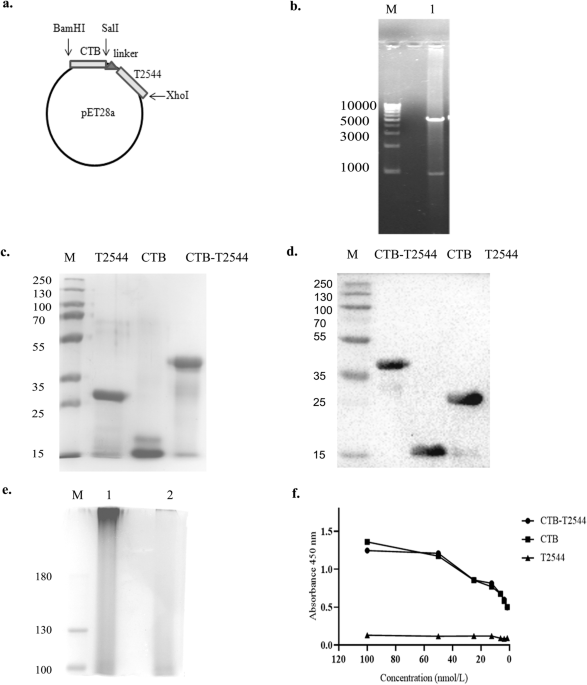
Approach- Here, in the chimeric formulation, CTB served as a mucosal adjuvant to the protein antigen, T2544. CTB was genetically fused with recombinant Salmonella Typhi outer membrane protein, T2544 through a non-furin linker (GPGP) and co-expressed and purified using Ni2+ affinity chromatography. CTB was chosen due to its non-toxic nature, ease of production as a recombinant fusion protein in large quantities in E. coli and rapid renaturation to the pentameric form of the native counterpart, enabling it to bind to its cognate GM1 ganglioside receptor2 and allowing the partner antigen to be taken up by the immune cells. In agreement with the previous studies, CTB and T2544, administered as a single, recombinant fusion protein through the intranasal mucosal route significantly induced T2544-specific humoral and cell-mediated immunity, not only at the local site, but also at distal mucosa, a phenomenon called ‘common mucosal immunity3,4.
Results- Intranasal administration of 60 µg of CTB-T2544 to an iron-overload mouse model protected 70% of the immunized mice against a lethal challenge with S. Typhi. These mice had markedly increased rT2544-specific IgG in serum and IgA in the intestinal wash and fecal extracts. These results were in agreement with the recovery of substantial numbers of T2544-specific IgG and IgA antibody-secreting cells (ASCs) by ELISpot assays from the spleen, mesenteric lymph nodes and and Peyer’s Patches (PP) of the small intestine. Protective efficacies of the serum and secretory antibodies were revealed by the inhibition of S. Typhi adherence to the intestinal epithelial cell monolayers, impairment of bacterial motility in the soft agar and the promotion of opsanophagocytosis. A lethal dose with S. Typhi, pre-incubated with intestinal lavage and fecal extracts from the immunized mice killed only 50% of the animals, while a sublethal dose of these bacteria showed significant reduction in the intestinal colonization abilities and spread to other visceral organs. These findings highlighted a significant role of mucosal antibodies in imparting protection. Moreover, intranasal CTB upregulates the expression of IgA-inducing factors on the lung resident dendritic cells and promotes their migration to the MLNs, where they imprint gut homing receptors on the lymphocytes, thus driving them to the intestinal mucosa to execute their effector function by sIgA and cytokines production. We found greater number follicular helper T (TFH) cells in the MLN, which play a crucial role in the germinal center formation and the development of high affinity antibodies and memory B cells as well as more IFNγ- and IL-17A-secreting cells in the Peyes patches. Collectively, these data indicated towards a protective milieu in the intestine with the induction of various T cell subsets and cytokines. Finally, we observed memory B and T cells in CTB-T2544 immunized mice that suggested long term immunity against S. Typhi infection.
Key takeaways- In a nutshell, we studied the immune adjuvant function of CTB to the protein subunit vaccine candidate, T2544 derived from Salmonlla Typhi for intestinal mucosal immune response after intranasal immunization.
Recombinant CTB-T2544 chimera induced:
(i) high titers of antigen-specific and protective serum IgG and mucosal sIgA antibodies.
(ii) sIgA response was mediated by CTB-elicited migration of DCs to the MLN, which expressed IgA-inducing factors and imprinted of gut homing receptors on the lymphocytes.
(iii) It highlighted the role of CTB in inducing a balanced T helper cell response, including the Th1, Th2, Th17 and TFH cells.
(iv) This, accompanied by augmented, antigen-specific memory B and T cell response after intranasal CTB elicited a strong and durable mucosal immunity at the intestine, leading to protection against oral S. Typhi infection.
References-
- Chakraborty, S., Dutta, P., Pal, A. et al. Intranasal immunization of mice with chimera of SalmonellaTyphi protein elicits protective intestinal immunity. npj Vaccines 9, 24 (2024).
- Stratmann, T. Cholera Toxin Subunit B as Adjuvant--An Accelerator in Protective Immunity and a Break in Autoimmunity. Vaccines (Basel) 3, 579-596 (2015).
- Holmgren, J. & Czerkinsky, C. Mucosal immunity and vaccines. Nat Med 11, S45-53 (2005).
- Sassone-Corsi, M. et al. Siderophore-based immunization strategy to inhibit growth of enteric pathogens. Proc Natl Acad Sci U S A 113, 13462-13467 (2016).
Follow the Topic
-
npj Vaccines

A multidisciplinary journal that is dedicated to publishing the finest and high-quality research and development on human and veterinary vaccines.
Related Collections
With Collections, you can get published faster and increase your visibility.
Therapeutic HPV vaccines
Publishing Model: Open Access
Deadline: Jun 30, 2026
Lipid nanoparticle (LNP)-adjuvanted vaccines
Publishing Model: Open Access
Deadline: May 19, 2026
Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in