Sustaining Immunity against Rabies: Insights from Cambodia's Post-Exposure Prophylaxis Protocol
Published in Biomedical Research
Rabies, one of the oldest known zoonotic diseases, remains a significant global health challenge despite being entirely preventable. This fatal viral disease claims an estimated 59,000 lives annually, with most cases occurring in Asia and Africa. Often overlooked due to its near invisibility in wealthier countries, rabies disproportionately affects rural, resource-limited settings where access to healthcare and awareness is limited.
Our recent study, conducted at the Institut Pasteur du Cambodge (IPC), investigates the durability of immunity provided by post-exposure prophylaxis (PEP) following dog bites. This work aligns with global efforts to eliminate rabies by 2030, an ambitious goal spearheaded by the World Health Organization (WHO) and supported by partners such as the Global Alliance for Vaccines and Immunization (GAVI).
This post shares our findings and their implications for achieving "Zero by 2030."
The Rabies Challenge: Why Cambodia's Fight Matters
In Cambodia, rabies is a public health emergency. The country reports an estimated 425,000 dog bite incidents annually, leading to approximately 800 human deaths. Children, particularly in rural areas, bear the brunt of these preventable fatalities. Despite the scale of the problem, public awareness remains low, and many individuals delay or forego life-saving PEP due to financial and logistical constraints.
At the heart of Cambodia’s response is the IPC Rabies Prevention Center, which administers PEP to thousands of patients each year. The center also employs the vaccine-sparing IPC protocol, an innovative regimen developed to maximize impact in resource-limited settings. Unlike traditional intramuscular protocols requiring several visits and higher vaccine volumes, the IPC protocol involves three intradermal doses over seven days, reducing both cost and patient burden.
Our Study: Measuring Long-Term Immunity
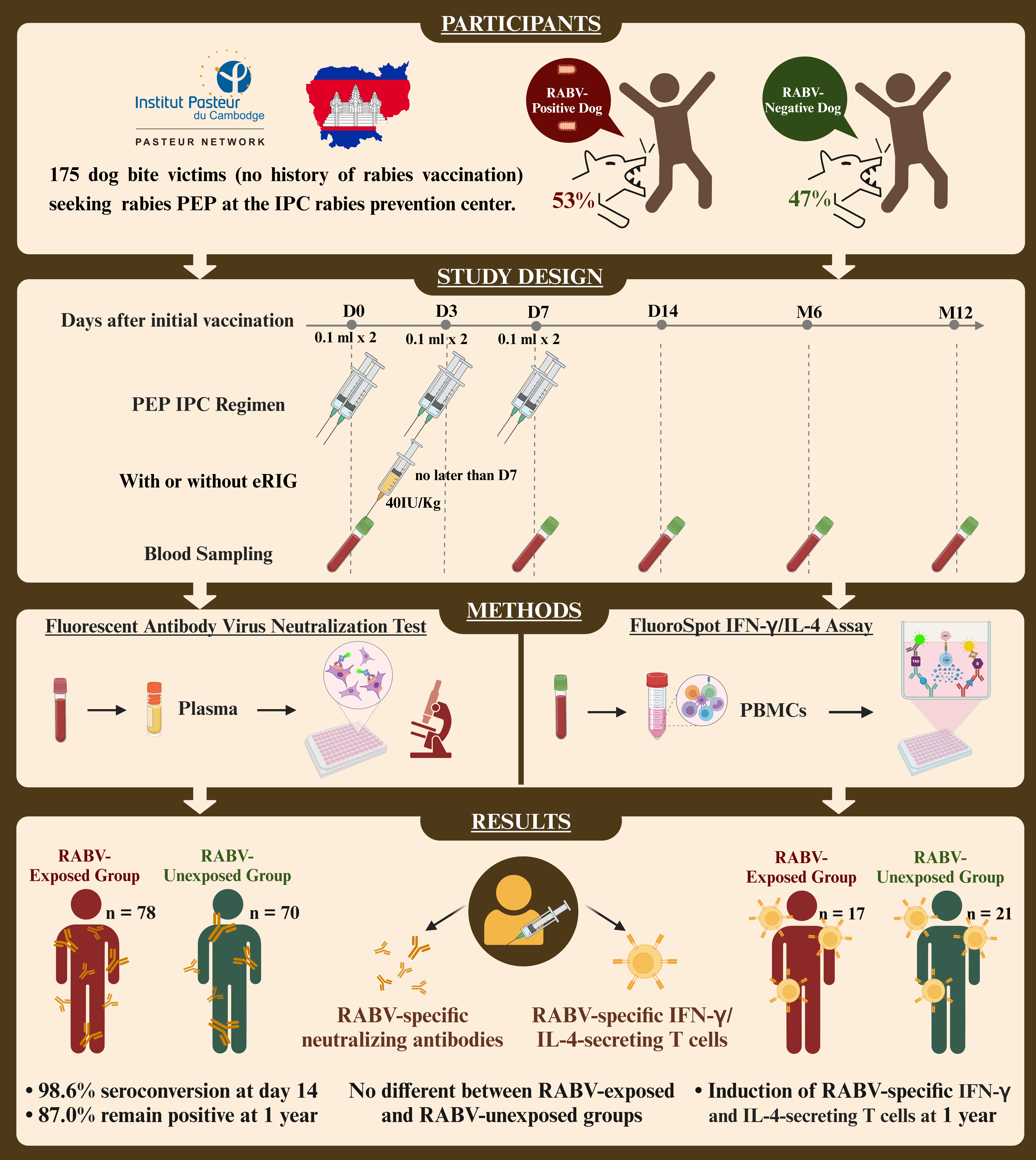
The primary aim of our study was to evaluate the durability of immune responses induced by the IPC regimen. We recruited 148 participants, all of whom were dog bite victims with no history of rabies vaccination. The cohort was divided into those exposed to rabies-positive dogs (and administered rabies immunoglobulin, eRIG) and those bitten by rabies-negative or untested dogs.
We analyzed neutralizing antibodies (nAbs) and T cell responses at five timepoints: baseline, 7 days, 14 days, 6 months, and 12 months after PEP.
Key Findings:
- Neutralizing Antibodies: Almost all participants developed protective antibody titers (≥0.5 IU/mL) 14 days post-PEP. One year later, 87% retained these protective levels, demonstrating the regimen's robust and enduring effectiveness.
- T Cell Responses: Both IL-4- and IFN-γ-secreting T cells showed significant increases at 14 days, persisting for up to a year. This sustained cellular response underscores the role of long-term immune memory in rabies protection.
Interestingly, no significant differences were observed between participants exposed to rabies-positive dogs and those unexposed, highlighting the protocol's consistent performance.
A Global Perspective: "Zero by 2030" and the GAVI Initiative
The results of this study take on added importance in the context of the global goal to eliminate human rabies deaths by 2030. Achieving this target, dubbed "Zero by 2030," requires a coordinated strategy that integrates dog vaccination, accessible PEP, and community education.
Recent efforts by GAVI to include human rabies vaccines in their portfolio mark a transformative moment for rabies control. By subsidizing vaccines for low- and middle-income countries, GAVI aims to ensure equitable access to life-saving PEP, addressing the financial barriers that currently prevent timely treatment.
The IPC regimen, which uses significantly less vaccine, complements this approach by maximizing vaccine availability while reducing costs. Scaling up vaccine-sparing protocols in endemic countries could play a crucial role in bridging the gap between resources and need, especially in remote or underserved regions.
The Broader Implications of Immunity Research
The persistence of immune responses observed in our study also has implications for future PEP guidelines. Rabies incubation periods can vary widely, occasionally extending beyond a year. The one-year immunity conferred by the IPC protocol provides critical protection during this window, but further research is needed to determine whether protective immunity can extend beyond this timeframe.
Our findings suggest that cellular immunity, particularly IFN-γ and IL-4-secreting T cells, plays a vital role in long-term protection. This opens new avenues for exploring vaccine formulations and adjuvants that enhance cellular responses, potentially improving both pre- and post-exposure vaccination strategies.
Challenges and Lessons from the Field
Conducting this research in a resource-limited setting like Cambodia posed unique challenges. The COVID-19 pandemic disrupted data collection, and logistical hurdles in reaching remote participants tested our resilience. These difficulties underscore the importance of tailoring research to local contexts and ensuring community engagement throughout the process.
Despite these obstacles, our real-world cohort offered invaluable insights. Unlike controlled laboratory settings, the diverse demographics and environmental conditions of our participants reflect the complexities of rabies prevention on the ground.
Moving Forward: A Call to Action
Rabies remains a stark reminder of the inequities in global health. Eliminating this preventable disease by 2030 requires sustained investment, collaboration, and innovation. Key priorities include:
- Scaling Up Dog Vaccination: With dogs serving as the primary reservoir for rabies, achieving high vaccination coverage in canine populations is non-negotiable.
- Improving Access to PEP: Vaccine affordability, supply chain improvements, and innovative protocols like the IPC regimen must be prioritized.
- Community Education: Awareness campaigns are vital for ensuring timely PEP administration and dispelling myths about rabies transmission.

As researchers, we also recognize the need to continue refining our understanding of rabies immunity. Our ongoing investigations aim to evaluate the IPC protocol’s long-term efficacy beyond one year and explore factors—such as genetics and environmental influences—that may affect immune responses.
Conclusion: Building Momentum toward "Zero by 2030"
Our study provides compelling evidence of the IPC regimen’s effectiveness in sustaining immunity against rabies for at least one year. These findings, coupled with initiatives like GAVI’s vaccine support, offer hope for achieving a world free from rabies deaths.
But success depends on translating research into action. Governments, international organizations, and communities must work together to close the gap between the tools we have and their equitable implementation. Only then can we realize the vision of a rabies-free future—one where no child needlessly loses their life to this preventable disease.

Follow the Topic
-
npj Vaccines

A multidisciplinary journal that is dedicated to publishing the finest and high-quality research and development on human and veterinary vaccines.
Related Collections
With Collections, you can get published faster and increase your visibility.
Lipid nanoparticle (LNP)-adjuvanted vaccines
Publishing Model: Open Access
Deadline: Feb 19, 2026
Therapeutic HPV vaccines
Publishing Model: Open Access
Deadline: Jun 30, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in