Paradigm Shift in Vaccinology: Harnessing Cellular Immunity with Thermostable Self-amplifying mRNA Vaccine
Published in Bioengineering & Biotechnology

COVID-19 pandemic and rise of our self-amplifying mRNA (SaRNA) platform technology
When the COVID-19 pandemic struck, the world faced an unprecedented health crisis. Healthcare systems were overwhelmed, and millions of people were at risk. The need for a solution was urgent, but traditional vaccine development, while effective, was too slow to keep up with the scale of the pandemic. We recognized the need to think innovatively to address this crisis meaningfully. This led us to explore SaRNA technology. The success of mRNA vaccines developed by Pfizer-BioNTech and Moderna not only helped curb the spread of COVID-19 but also showcased the potential of mRNA technology for future vaccine development. Alongside this, the concept of using messenger RNA to instruct cells to produce viral proteins and trigger an immune response emerged as a promising avenue. However, we aimed to push the boundaries even further. What if we could enhance the effects of the mRNA, making it more efficient and requiring a smaller dose? This is where Gennova’s SaRNA platform came into play. Unlike traditional mRNA vaccines, our SaRNA technology enables the mRNA to replicate within cells, producing more viral proteins and eliciting a stronger immune response with less material. This approach not only made the vaccine more potent but also allowed for a more scalable and cost-effective solution.
Development of the Thermostable Monovalent SaRNA Vaccine GEMCOVAC-OM for Equitable Access
We developed the thermostable monovalent SaRNA vaccine, GEMCOVAC-OM, to ensure equitable access to effective mRNA vaccines, especially for low- and middle-income countries (LMICs). One of the main challenges we identified was the increased degradation of mRNA in water, likely due to hydrolysis. To address this issue, we implemented a freeze-drying (lyophilization) process for the vaccine. This innovation extends the shelf life of GEMCOVAC-OM and enables it to be stored and transported at higher temperatures, eliminating the need for complex ultra-cold chain systems. By mitigating logistical challenges and reducing costs, GEMCOVAC-OM can reach remote and underserved areas with limited healthcare infrastructure, thereby improving vaccine coverage and equity. Furthermore, we chose to develop a monovalent Omicron booster vaccine due to the phenomenon of antigenic sin, or immune imprinting. This approach aims to elicit a more variant-specific immune response across diverse populations.
Intradermal Delivery of GEMCOVAC-OM to Maximize Cellular Immune Responses for Enhanced Immunological Breadth and Durability
The dermal layer contains a dense network of cells, including dendritic cells, macrophages, and T-cells. This cellular richness allows intradermal vaccination to produce a robust and comprehensive immunogenic response compared to intramuscular administration. Additionally, the self-replicating nature of SaRNA mimics a natural infection, providing stable and sustained delivery of the antigen within host cells, which further enhances the immune response. These benefits, combined with the use of the intradermal delivery route, motivated us to examine the cellular outcomes of GEMCOVAC-OM in clinical settings, ensuring its effectiveness against various SARS-CoV-2 variants. For this study, we administered the GEMCOVAC-OM vaccine intradermally using the needle-free Tropis® injection system.
Study Outcomes
GEMCOVAC-OM has been developed as a next-generation mRNA vaccine. A post-hoc analysis from a multicentred, randomized phase-3 study allowed us to evaluate its cellular immune breadth when administered as a booster. In this analysis, we compared GEMCOVAC-OM— which encodes the Omicron B.1.1.529 Spike protein— with the widely used adenovector vaccine ChAdOx1 nCoV-19, which encodes the Wuhan variant Spike protein. Using ChAdOx1 as a benchmark enabled us to directly assess how GEMCOVAC-OM performed against a vaccine that was already extensively deployed in India during the study period. The results were promising: GEMCOVAC-OM induced a significant expansion of memory B cells (MBCs) specific to Omicron B.1.1.529 compared to ChAdOx1 nCoV-19. Additionally, GEMCOVAC-OM triggered a greater number of B cells reactive to other Omicron variants, including XBB.1.5 and BA.2.86 Spike proteins. Beyond B cells, GEMCOVAC-OM also stimulated higher frequencies of Omicron-Spike-specific T cells, including polyfunctional cells, stem cells, and both central and effector memory subsets. While ChAdOx1 nCoV-19 exhibited some cross-reactivity, GEMCOVAC-OM elicited a more targeted and robust immune response, indicating its superior ability to provide broader and longer-lasting immunity. In summary, GEMCOVAC-OM stands out for its promising ability to induce a comprehensive immune response, positioning it as a potential candidate for future vaccine development and global distribution.
A Paradigm Shift in Vaccinology
The cellular responses observed with the SaRNA platform-based vaccine, GEMCOVAC-OM, present a transformative opportunity for the future development of vaccines against infectious diseases and cancer (Figure).
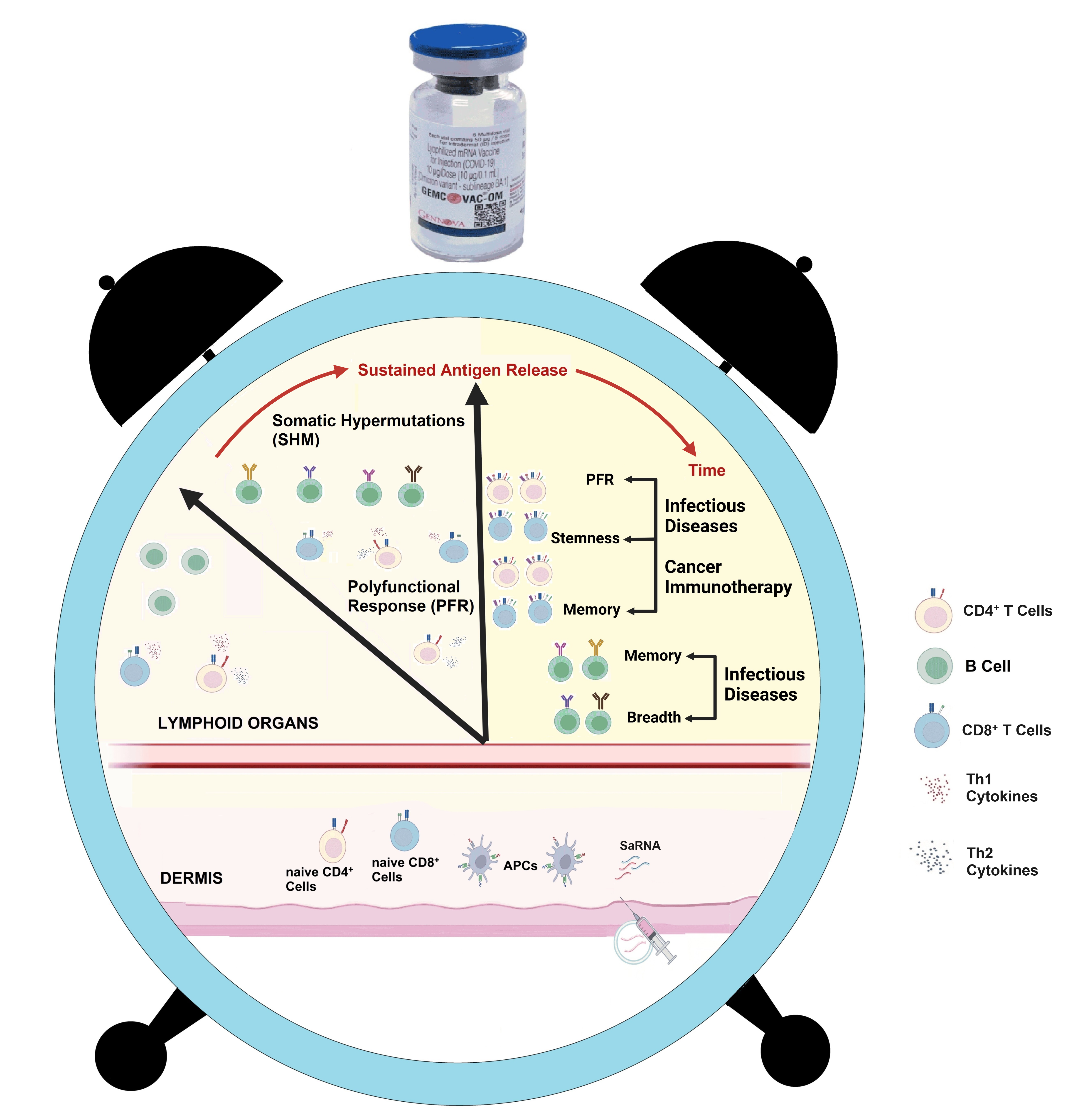
The strong breadth of B-cell responses and the polyfunctional T-cell responses demonstrated by GEMCOVAC-OM underscore the platform's capability to elicit a comprehensive immune reaction. This is particularly crucial for combating highly mutating variants, such as those seen in SARS-CoV-2. Moreover, the enhanced memory T-cell responses, particularly the T-cell stemness observed, further highlight the platform's potential for providing long-lasting immunity. For infectious diseases, the SaRNA platform's ability to generate broad immune activation may help overcome challenges posed by the rapid mutation of pathogens, ensuring protection across a wide range of variants. Additionally, the platform's potential to induce targeted and durable immune responses could offer significant benefits in the development of vaccines for complex diseases like HIV and TB, where traditional methods have often failed to deliver sufficient immunity. In the context of cancer immunotherapy, the platform's ability to generate strong and diverse immune responses—including polyfunctional T-cells and memory T-cells—opens the door to developing vaccines that stimulate anti-tumor immunity. This could potentially revolutionize cancer treatment strategies. By achieving critical correlates of protection, SaRNA vaccines have the capability to shift the paradigm for vaccine development across these diverse and challenging fields.
Follow the Topic
-
npj Vaccines

A multidisciplinary journal that is dedicated to publishing the finest and high-quality research and development on human and veterinary vaccines.
Related Collections
With Collections, you can get published faster and increase your visibility.
Lipid nanoparticle (LNP)-adjuvanted vaccines
Publishing Model: Open Access
Deadline: Feb 19, 2026
Therapeutic HPV vaccines
Publishing Model: Open Access
Deadline: Jun 30, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in