Antibody-displaying extracellular vesicles for targeted cancer therapy
Published in Bioengineering & Biotechnology and Cancer
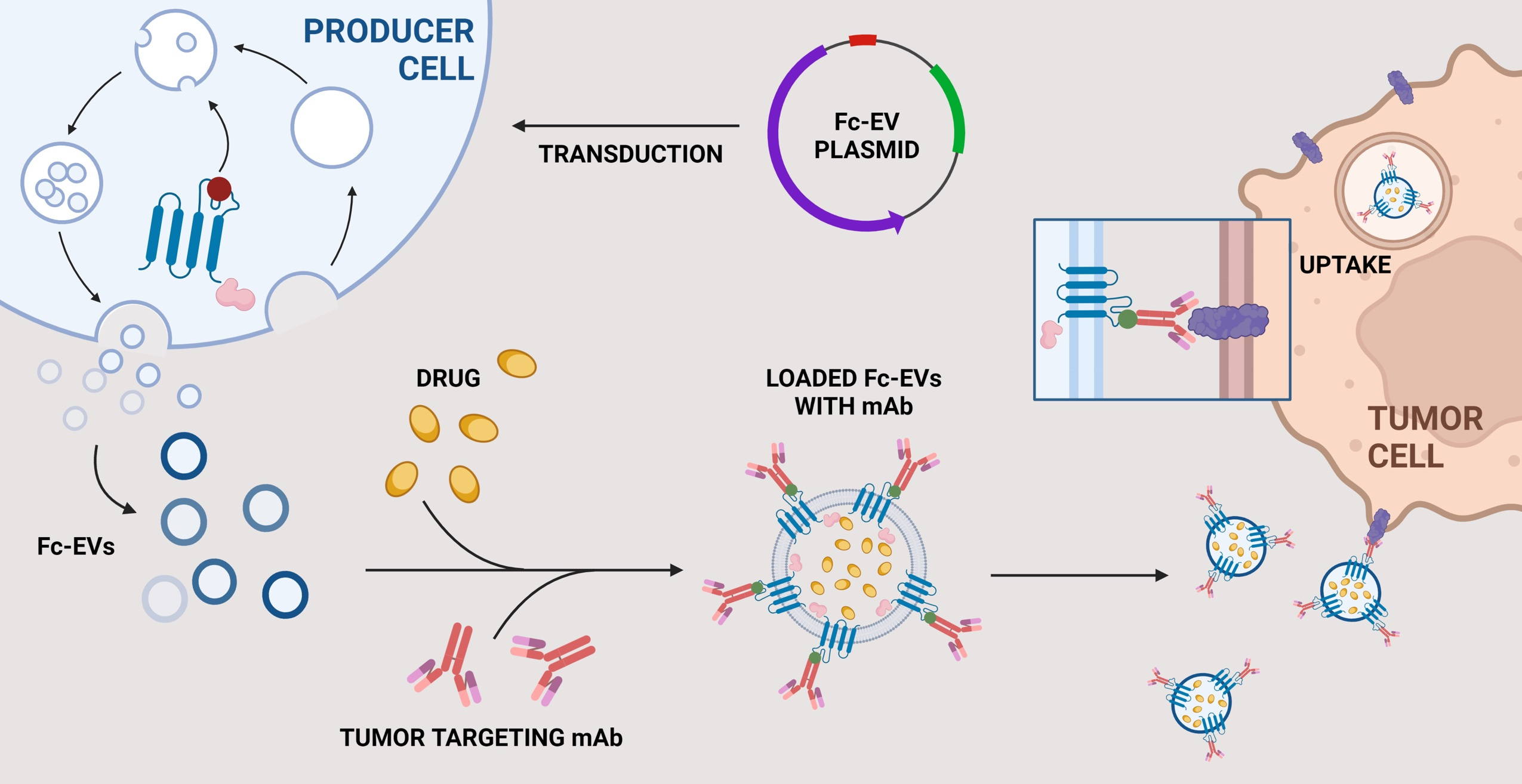
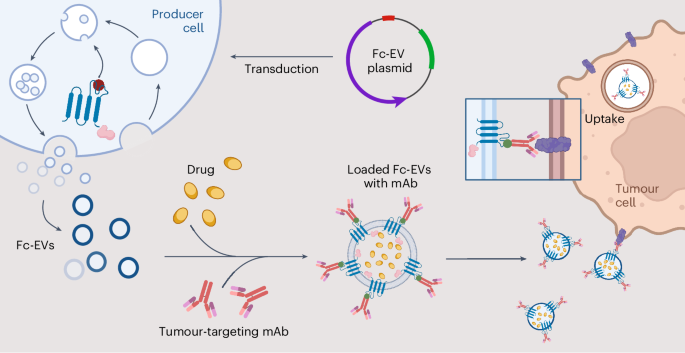
EVs are increasingly being investigated as promising biotherapeutics owing to their potential as a natural delivery vector that can transport macromolecular content and surpass biological barriers to the site of interest. EVs are associated with minimal toxicity and improved drug potency, with enhanced tumour penetrance, and retention in tumour cells. EVs can be bioengineer to express targeting moieties, which we and others have reported previously. Here, the aim was to develop a versatile system by displaying antibodies. Our hypothesis was that this would be beneficial compared to conventional EV targeting systems as the antibodies here can be interchanged depending on the intended target, thereby offering the potential to utilize Fc-EVs as an off-the-shelf modular drug that can be combined with previously approved therapeutic antibodies.
In our recent publication in NBME, we performed a screen of 9 Fc-binding domains and 9 EV sorting domains to optimize the antibody display. We show that Fc-EVs can be used to enrich EVs by several 100-folds to a cellular target, such as the therapeutically used targets PD-L1 and HER2, both in vitro and in vivo. We also showcased that EVs displaying PD-L1-Ab have a significantly enriched and sustained accumulation in tumour tissue up to 72 hours after one single intravenous injection. Furthermore, when loaded with chemotherapeutic drugs, EVs displaying PD-L1-Ab resulted in an improved anti-tumoral effect with prolonged survival and decreased tumour progression.
We believe that the Fc-EV technology has great potential as it offers the possibility to combine EV-antibody therapy in which targeting and/or therapeutic antibodies facilitate targeted EV delivery with therapeutic cargo that can generate a synergistic effect. In theory, the technology could be applied to multiple diseases and used in multiple combinations, not only limited to classical antibody but could also include e.g. Fc-fused proteins, antibody-drug conjugates and bi-specific antibodies. Subsequent studies on the Fc-EV concept will hopefully explore the therapeutic potential further and possibly translate it to clinical use.
Please check out our publication and let us know what you think.
Follow the Topic
-
Nature Biomedical Engineering

This journal aspires to become the most prominent publishing venue in biomedical engineering by bringing together the most important advances in the discipline, enhancing their visibility, and providing overviews of the state of the art in each field.
Related Collections
With Collections, you can get published faster and increase your visibility.
Biosensing
Publishing Model: Hybrid
Deadline: Mar 26, 2026
Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in
and here is the link: https://www.nature.com/articles/s41551-024-01214-6