Behind the paper Regulation of cortical neurogenesis by MED13L via transcriptional priming and its implications for MED13L syndrome
Published in Neuroscience
After many years of investigating the molecular and cellular mechanisms that shape the brain’s remarkable structure during development, our understanding of brain morphogenesis steadily deepened. Yet, alongside this growing knowledge came an ever-intensifying curiosity and concern about what happens when these developmental processes go wrong.
Neurodevelopmental disorders are alarmingly diverse and widespread: autism spectrum disorder, attention deficit hyperactivity disorder, intellectual disability, Rett syndrome, and many others. While we know that most of these conditions are caused by genetic mutations that disrupt brain structure and function, it is frustrating that the underlying mechanisms remain largely unknown. Even more troubling, effective treatments for these disorders are still lacking.
“The urgency of this issue became personal. Five of my close friends have children diagnosed with some form of neurodevelopmental disorder. As a developmental neurobiologist, I began to feel an increasing sense of dissatisfaction—an inner voice that kept asking: What can we do, as basic scientists, to help these children and their families?”
recalled Dr. Xiaobing Yuan.
“In 2018, a pivotal opportunity emerged. We connected with Dr. Xuelian He, a pediatric geneticist at Wuhan Children's Hospital. I still vividly recall our first conversation. She told me, with both clarity and concern, that although clinicians can now accurately diagnose many neurodevelopmental disorders and even identify the underlying gene mutations, they often have little understanding of how those mutations cause disease.”
That gap—between clinical genetics and mechanistic insight—was precisely where our expertise could make a difference.
We decided to collaborate, aiming to bridge this divide by uncovering how specific gene mutations translate into the phenotypes observed in patients. Our first target was a disorder that Dr. He frequently encountered in the clinic but for which little mechanistic knowledge existed: MED13L syndrome. She noted that they had seen multiple cases in the Chinese population. Affected children displayed distinctive facial features, intellectual disability, motor impairments, ataxia, and, in some cases, autistic behaviors.
As developmental neurobiologists, we recognized that a gene knockout mouse model would be an invaluable tool to explore how Med13l dysfunction affects brain development and behavior. And so, this line of research began.
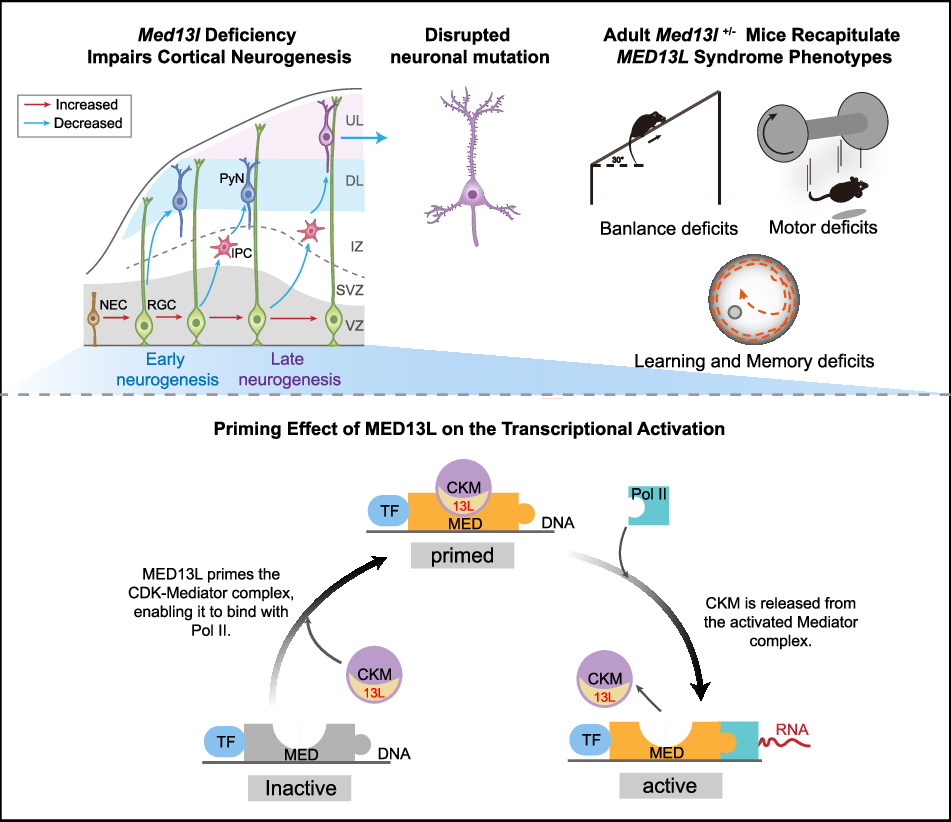
With the help of a biotechnology company, we used gene-editing technology to generate Med13l knockout mice. “When we analyzed the model, I was greatly encouraged to find that the mice recapitulated many of the core symptoms observed in patients—intellectual and motor impairments—and also displayed microcephaly,” said Jia Lian, the first author of this article. Brain imaging and histological analysis revealed significant volume reduction in the cerebral cortex, cerebellum, and striatum—regions critically involved in cognition and motor function. These results strengthened our resolve to use this model to investigate the mechanisms driving MED13L syndrome.
Naturally, more questions followed: What causes these structural brain abnormalities? How do they link to behavioral symptoms? Our study found that the cortical thinning originated from disrupted embryonic neurogenesis. Moreover, in adult heterozygous knockout mice, we observed pronounced defects in neuronal morphology and dendritic spine density—cellular abnormalities that impair connectivity and persist from development into adulthood. These findings offered a direct mechanistic link between gene mutation and the observed intellectual and motor impairments.
This project took seven years of dedicated effort. “The early stages were particularly challenging,” Jia recalled. “We had to verify gene knockout efficiency, generate and validate custom antibodies, and navigate the many unknowns of a new disease model. We faced all kinds of technical hurdles—optimizing brain tissue sectioning, perfecting staining protocols for high-resolution imaging, and fine-tuning Co-IP conditions to identify reliable protein interactors. Each step was taken with care to ensure the rigor and reproducibility of our data.” Under the leadership of Dr. Xiaobing Yuan, the entire team worked tirelessly, with each member contributing their expertise to lay the foundation for this novel line of research.
We are deeply gratified that this work has now been published and shared with the scientific community. But for us, this is only the beginning. MED13L syndrome still holds many secrets. We remain committed to uncovering its mysteries—translating our basic research findings into insights that will help clinicians, families, and ultimately, patients. We hope that our work will one day contribute to new intervention strategies and therapeutic targets for this devastating disorder.
Follow the Topic
-
Communications Biology

An open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of the biological sciences, representing significant advances and bringing new biological insight to a specialized area of research.
Related Collections
With Collections, you can get published faster and increase your visibility.
Signalling Pathways of Innate Immunity
Publishing Model: Hybrid
Deadline: Feb 28, 2026
Forces in Cell Biology
Publishing Model: Open Access
Deadline: Apr 30, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in