Stress landscape of folding brain serves as a map for axonal pathfinding
Published in Bioengineering & Biotechnology, Neuroscience, and Anatomy & Physiology
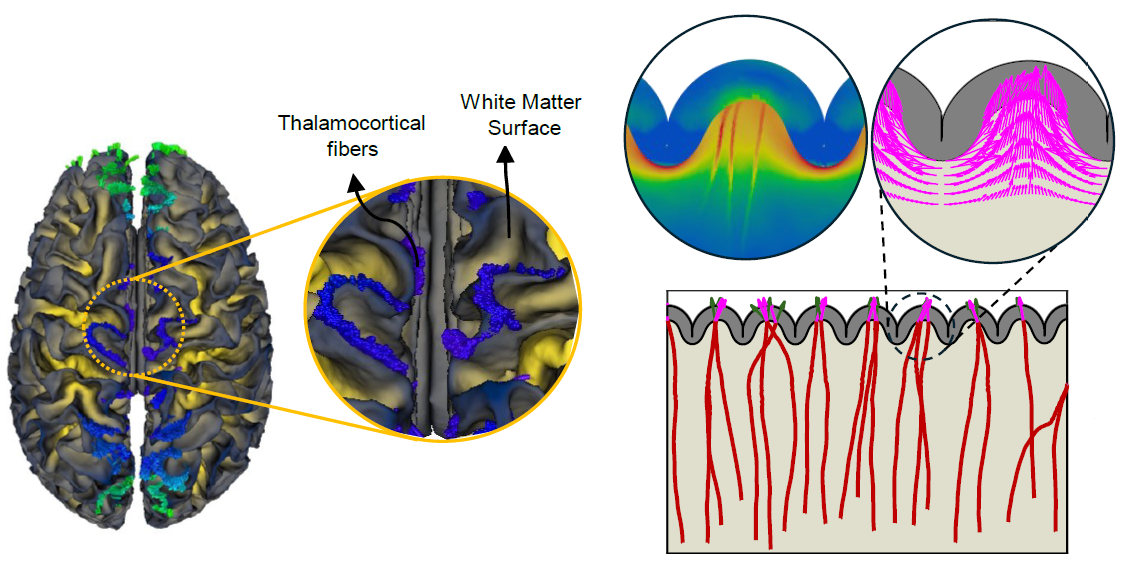
Introduction
This study introduces the theory of "axon reorientation" and develops a mechanical model to elucidate how growing axons navigate within the folding brain. The model simulates the physical interplay between cortical folding, connectivity development, and patterning. This Behind the Paper article aims to present the main goals and findings of the research in simple language. For technical details, readers can refer to the published paper and supporting references.
Why is understanding the link between brain folding and connectivity development important?
The emergence and development of brain connectivity fiber tracts occur predominantly alongside cortical folding. During early brain development, axonal fibers extend to form connections between different regions of the brain. These fiber tracts grow bidirectionally—extending from the cortex to the white matter and from the white matter to the cortex. As the cortex begins to fold, these fibers navigate through the evolving landscape of gyri and sulci. Understanding the interaction between cortical folding and connectivity development is essential for unraveling the complexities of brain formation and function.
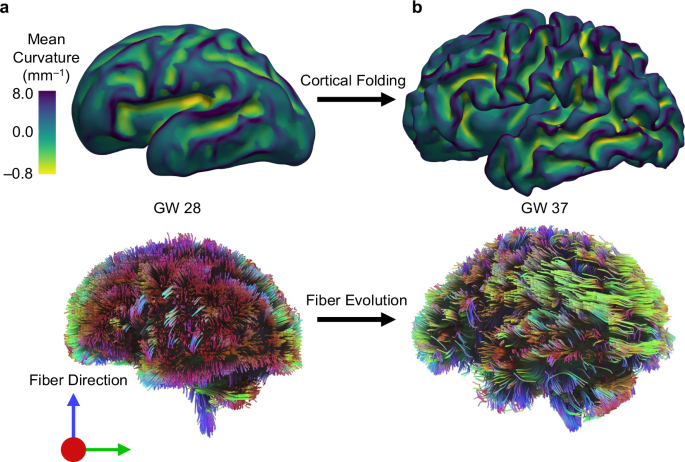
Cortical folding increases the brain’s surface area, enabling more efficient neural connectivity and enhanced cognitive processing. During development, axons grow and establish connections while navigating the dynamic landscape of gyri and sulci, highlighting the close relationship between folding and connectivity. Disruptions in this interplay are associated with neurodevelopmental disorders such as autism spectrum disorder (ASD), schizophrenia, and bipolar disorder. By comprehending the physical and mechanical factors guiding axon growth in the folding brain, we can gain critical insights into the mechanisms underlying normal and abnormal brain development. Ultimately, this understanding contributes to improved diagnostics and therapeutic strategies for these conditions. Figure 1 illustrates the progression of growth and folding in an individual fetal brain, transitioning from a smooth state to a folded state between two developmental time points. Concurrently, brain connectivity also evolves during this period, which is closely intertwined with the folding of the cortical plate.
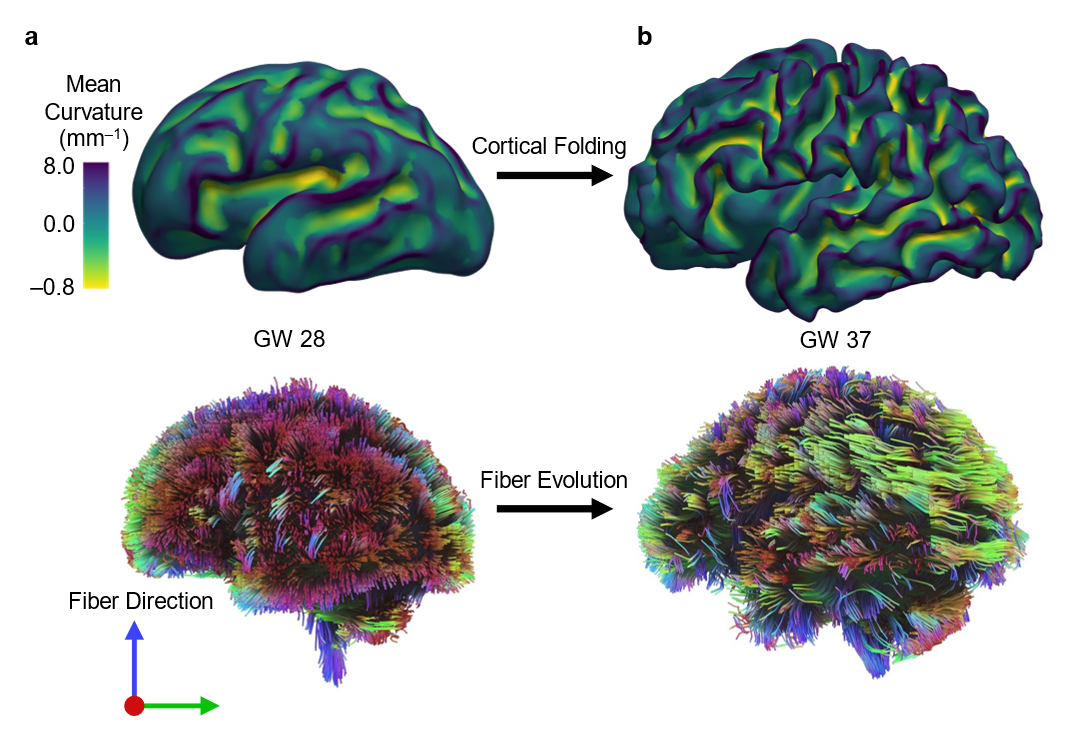
Current knowledge gap in the mechanics of brain folding
The brain’s intricate folds have intrigued scientists for years, with various theories attempting to explain their formation. Among them, differential tangential growth suggests that the outer brain layers grow faster than the inner layers, driving cortical folding. This idea, supported by both experiments and simulations, highlights differential tangential growth as a key factor in shaping secondary and tertiary folds.
Axonal maturation has also been proposed as a contributor, but recent findings suggest that rather than axons pulling the brain into folds, the folding cortex influences axonal elongation and white matter growth. While efforts have been made to model axonal forces in brain development, the dynamic relationship between folding and connectivity remains poorly understood. This knowledge gap is significant because altered folding patterns correlate with conditions like ASD, schizophrenia, and bipolar disorder. Disruptions in connectivity and folding may originate as early as fetal development, yet it remains unclear whether connectivity changes drive folding abnormalities or vice versa. Bridging this gap could provide deeper insights into neurodevelopmental disorders and brain function.
The proposed theory and its foundations
This study introduces the concept of axon reorientation in the folding brain and develops a mechanical model to uncover the multiscale mechanics linking cortical folding and connectivity development. During brain development, axons bundle together and grow along defined pathways to establish functional connections. Soft fibrous tissues, such as white matter, exhibit fiber alignment in response to mechanical loading, with fibers tending to orient along principal stress directions. Inspired by this, we proposed a model where axon bundles reorient and grow in alignment with the maximum tensile principal stress.
Unlike previous studies focusing on passive or continuum-based fibers, our framework captures the intricate behavior of axon bundles, including stress-induced and stochastic growth alongside reorientation. The concept of axon reorientation builds on experimental findings showing that neurites preferentially align with stretching. We hypothesize that, beyond stress-induced growth, axons reorient to form stereotyped subcortical fiber patterns. Our simulations, which integrate this complex axonal behavior, provide a more accurate representation of connectivity development and explain why gyri exhibit higher axon density than sulci, aligning with in vivo diffusion tensor imaging data. This model specifically examines axonal growth and pathfinding from white matter toward the cortical plate, such as thalamocortical tracts that emerge in early development and complete their formation by the third trimester, coinciding with cortical folding.
Summary of key findings of the study
- Axonal fibers concentrate more in gyri than sulci. Our imaging data reveal that axonal fibers, particularly those projecting from the brain core to the cortical plate, are more concentrated in gyri compared to sulci. This pattern holds for both thalamocortical tracts and fibers extending from the cortical plate toward deeper brain regions. Our findings align with prior studies on macaque and chimpanzee brains, reinforcing the idea that functionally complex regions predominantly localize in gyri. Figure 2 illustrates how fiber trajectories align with imaging findings, demonstrating that thalamocortical fibers predominantly exhibit radial alignment beneath gyri and tangential alignment beneath sulci, consistent across all cases analyzed. Additionally, the comparison of simulations with and without stress-induced reorientation highlights the crucial role of mechanical forces in guiding fiber organization, particularly at lower axon growth rates, where stress fields have sufficient time to influence fiber directionality toward gyri.
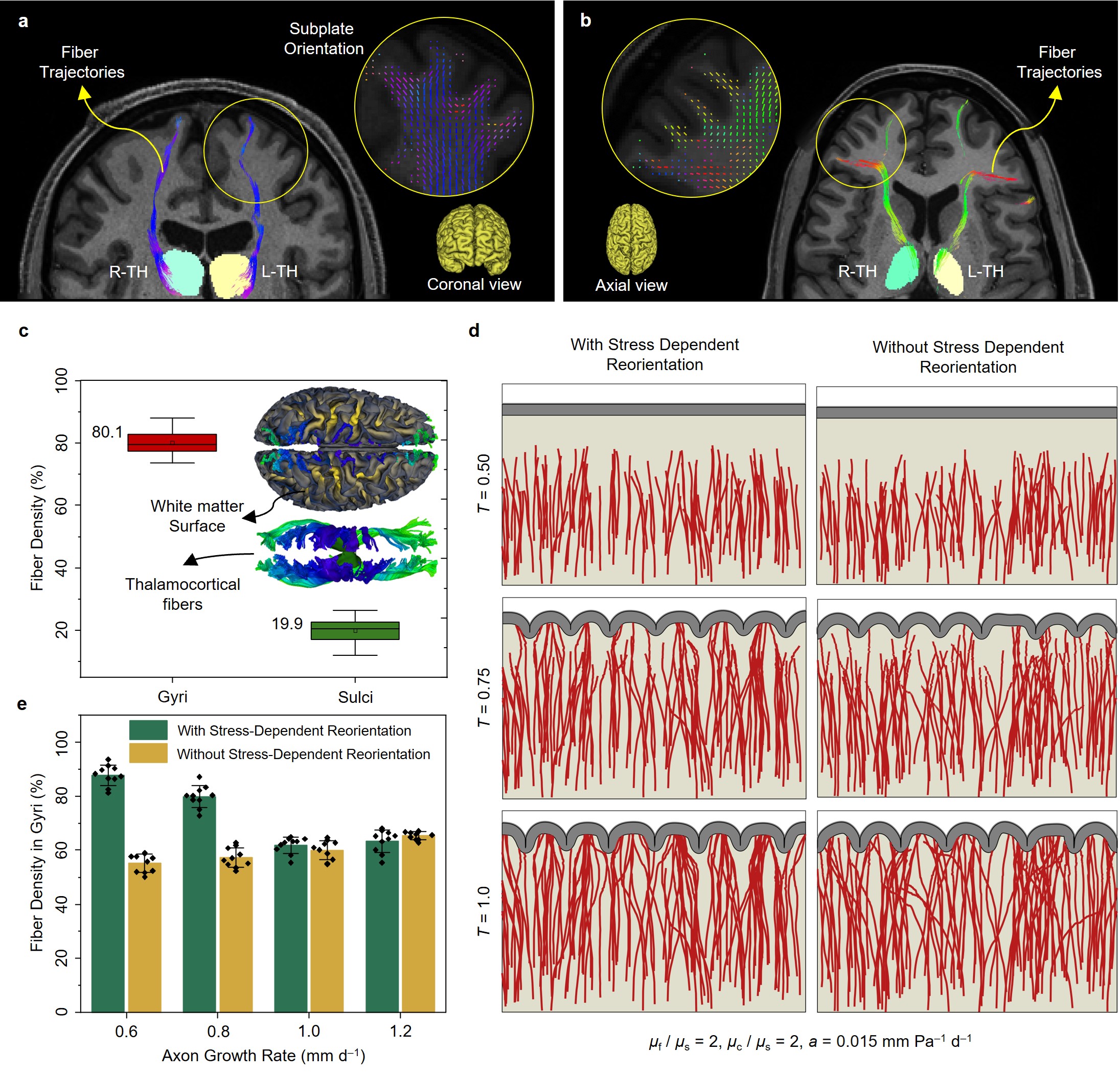
Fig. 2. Anisotropic fiber organization in the adult brain and the effect of stress-dependent fiber reorientation on connectivity patterning. a, b Fiber trajectories and orientation map (enclosed within yellow circles) based on high-resolution DTI imaging for (a) coronal view, and (b) axial view. As shown in the fiber trajectories, thalamocortical fibers mainly terminate in gyral regions. Within the subplate, fiber orientation beneath the sulci is predominantly tangential, while those within gyri are radial. Observations from subplate orientation suggest that fibers alter their trajectories upon nearing sulcal regions, preferring settlement within gyri. c Average fiber density in gyri and sulci of 10 brains for thalamocortical fiber tracts. The boxes represent the interquartile range (IQR), spanning the 25th to 75th percentiles, with the mean shown as a dot and the median indicated by a black line within the box. The whiskers depict the mean +/- SD. The analysis was conducted with a sample size of n = 10. Blue areas on the surfaces of the white matter indicate the tips of thalamocortical fibers. For better clarity, readers are referred to the color version of the figure in the online version of this article. The MRI and diffusion MRI images featured in this figure were sourced from the publicly available Q1 release of the WU-Minn Human Connectome Project (HCP) database (https://humanconnectome.org/). d Dynamic growth of fibers for the axon growth rate of Gaxn=0.6mmd−1 in models with and without stress-induced reorientation process. μf, μs, and μc are the shear moduli of the fiber, ECM, and cortex, respectively, and a is the stress-dependent elongation rate. e Comparison of fiber density within gyri for simulations conducted with and without the stress-dependent reorientation process, across different fiber growth rates. Error bars represent the mean +/- SD, while black dots show individual data points. The analysis was performed with a total sample size of n = 80, comprising 40 data points per group. Source data are provided as a Source Data file.
- Axonal growth is influenced by the stress field. Cortical folding creates a dynamic stress and deformation landscape that affects axonal growth rates and orientations. Tensile stresses beneath gyri facilitate axonal elongation, whereas compressive forces beneath sulci hinder growth and cause axons to change direction. Our model supports experimental observations that axons respond to mechanical cues, redirecting their trajectories when encountering resistance, much like axons navigating around obstructions in in vitro studies.
- Axonal tension aligns with cortical folding mechanics. Our results confirm that stretching towards gyri generates pulling forces in axons, consistent with experimental evidence showing that axons experience tension early in development and throughout connectivity formation. These observations align with previous studies on the ferret brain and diffusion tensor imaging-based findings in rhesus macaques. Unlike prior models that treated subplate development as a passive process, our findings emphasize the role of white matter heterogeneity in dictating folding patterns, with dense fiber regions forming gyri and sparse regions forming sulci.
- The relationship between cortical folding and connectivity development is bidirectional. We demonstrate that cortical gyrification and connectivity development influence each other dynamically. The folding process alters the stress landscape, shaping fiber growth, while axonal development affects the mechanical properties of the brain and contributes to fold positioning.
- Axonal growth rates and folding patterns influence brain developmental disorders. Our study confirms prior predictions that slower fiber elongation or faster cortical growth enhances gyrification, whereas the opposite reduces folding. These insights have direct implications for understanding polymicrogyria (characterized by excessive folds) and lissencephaly (marked by reduced folding), both of which exhibit connectivity disruptions.
- Axon biophysical properties affect fold placement and fiber organization. The interplay of deterministic mechanical interactions and stochastic biochemical influences governs axonal growth patterns during cortical folding. We find that increased randomness in axonal growth angles disrupts pathfinding and delays cortical connectivity establishment.
- Implications for brain disorders and future research directions. Our findings suggest that disruptions in axonal pathfinding and mechanical interactions could contribute to developmental brain disorders. Future studies could refine this model by incorporating additional factors such as biochemical signaling and dynamic white matter remodeling.
Behind the Paper: Research Group
We are a dedicated research group in the Department of Mechanical Engineering at the State University of New York at Binghamton (SUNY Binghamton), driven by a shared passion for uncovering the intricate mechanics of brain development. Over the past three years, our team (Dr. Mir Jalil Razavi, as Principal Investigator, and two PhD students, Akbar Solhtalab and Ali H. Foroughi) has worked together to develop a theoretical framework that bridges mechanical modeling and neurodevelopment.
Our journey was filled with challenges—trial and error, unexpected setbacks, and the complexity of integrating physics-based models with medical imaging. However, through relentless determination, rigorous problem-solving, and unwavering collaboration, we overcame these hurdles. The culmination of our efforts has led to findings that we believe offer a fresh perspective on how cortical folding influences connectivity and, ultimately, neurodevelopmental disorders.
This journey would not have been possible without the invaluable support and expertise of our esteemed collaborators. We extend our deepest gratitude to Dr. Lana Pierotich from Boston Children’s Hospital and Dr. Ali Gholipour from Boston Children’s Hospital and the University of California, Irvine. Their insights and contributions have played a crucial role in refining our models and enhancing the clinical relevance of our research.
As we continue to push the boundaries of understanding brain mechanics, we remain committed to scientific inquiry, interdisciplinary collaboration, and the pursuit of knowledge with the potential to improve lives.

Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Your space to connect: The Psychedelics Hub
A new Communities’ space to connect, collaborate, and explore research on Psychotherapy, Clinical Psychology, and Neuroscience!
Continue reading announcementRelated Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in