Crown-hydroxylamines
Published in Chemistry
Unlike amines, hydroxylamines are rarely used as ligands for transition metal coordination compounds. This is partially because of the instability of these complexes that undergo decomposition, disproportionation and oxidation involving the hydroxylamine motif (Fenton-like behavior). Indeed, the oldest known coordination compound of hydroxylamine, Zn(NH2OH)2Cl2 (Crismer's salt) releases NH2OH already at ambient temperature and detonates upon heating. Structurally characterized complexes in which the metal ion is coordinated by more than three hydroxylamine groups are scarce and unstable. On the other side, an intriguing feature of hydroxylamine ligands is their redox non-innocence which can be used to modulate electron transfer reactions. In particular, d-mental complexes of organic hydroxylamines are known to catalyze aerobic oxidation reactions. In these processes, the N−O bond can intramolecularly assist the oxidation of an organic substrate via nitroxyl radical species.
To overcome the stability issue, we designed and synthesized a novel family of macrocyclic chelating ligands, crown-hydroxylamines, which incorporate several N-OH units within one molecule. Crown-hydroxylamines form stable complexes containing a d-metal ion (Cu(II), Ni(II), Mn(II), and Zn(II)) coordinated by multiple (up to six) hydroxylamine fragments. The structure and stability of crown-hydroxylamines and their complexes are largely governed by strong intramolecular H-bonding interactions between the N−OH groups. Crown-hydroxylamine complexes exhibit pH-dependent behavior where the efficiency of metal binding increases upon deprotonation of the hydroxylamine groups. They also show promising catalytic activity in the aerobic oxidative activation of S−H and N−H bonds, while the corresponding macrocyclic amine complexes show poor performance in these reactions.
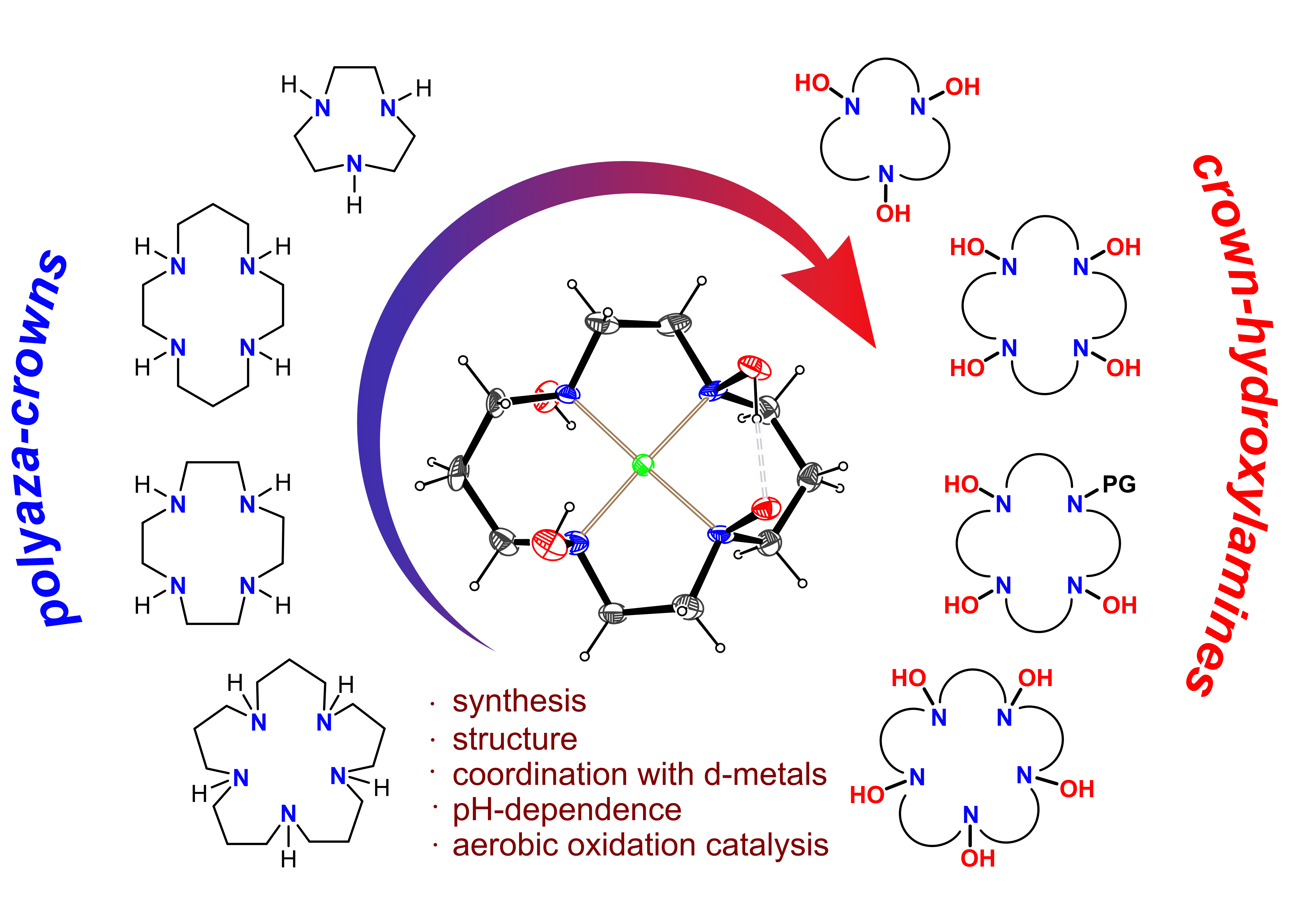
Overall, crown-hydroxylamines could become a good supplement to the variety of macrocyclic amine ligands available in the chemist’s toolbox.
To learn more about these fascinating molecules, check out our paper “Crown-hydroxylamines are pH-dependent chelating N,O-ligands with a potential for aerobic oxidation catalysis” in Nature Communications (https://www.nature.com/articles/s41467-023-43530-6).
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in