Delivering genes to mitochondria with a fluorinated modified tool
Published in Bioengineering & Biotechnology and Cell & Molecular Biology
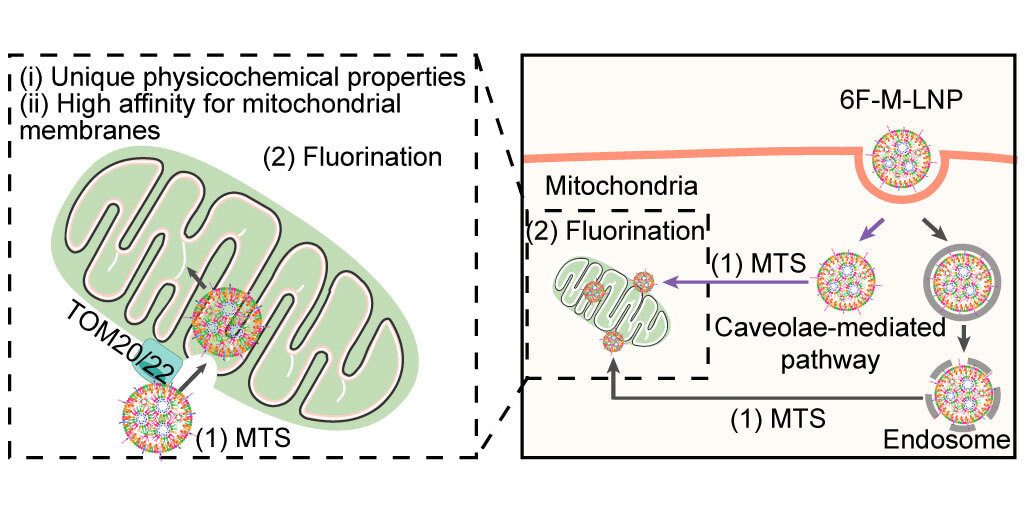
The challenge: Reaching the core of mitochondria
Mitochondria, the power plants of our cells, possess their own DNA (mtDNA). Mutations in mtDNA are the root cause of a range of devastating and currently incurable genetic disorders. In theory, gene therapy is the ultimate solution, aiming to deliver gene therapeutics into mitochondria for functional complementation or to correct these mutations. However, this is extremely difficult because the double membranes of mitochondria act as a double, fortified wall, that hinder the entry of exogenous genes. Moreover, in diseased cells, the mitochondrial membrane potential (MMP)—an electrical property that many delivery methods rely on—is often weakened. This creates a major barrier: how to effectively transport gene therapeutics into mitochondria without depending on MMP.
The approach: Harnessing the unique chemistry of fluorine
Existing delivery strategies primarily use positively charged molecules or mitochondrial targeting signals (MTS) that require a healthy MMP. We sought a different approach. Our inspiration came from studies showing that fluorinated compounds, due to their unique hydrophobic and lipophobic characteristic, can passively cross lipid membranes.
We hypothesized that incorporating fluorine into lipid nanoparticles could provide a new way to penetrate mitochondrial membranes. This mechanism, we predicted, would not depend on the MMP, making it potentially effective even in diseased mitochondria. We designed fluorinated lipid nanoparticles (F-LNPs) equipped with MTS peptides. The MTS acts as a guide to bring the nanoparticles to the mitochondria, while the fluorinated lipids are intended to facilitate the final step of crossing the membranes.
The findings: Efficient, MMP-independent delivery
The initial results were more encouraging than we had dared to hope. Our experiments demonstrated that these MTS-modified F-LNPs could indeed efficiently deliver genetic cargo into the mitochondrial matrix. Crucially, this success was not diminished in cells where we artificially collapsed the MMP. This confirmed that the fluorination strategy works independently of the mitochondrial energy state, overcoming a key limitation of previous methods. How were these nanoparticles actually achieving this feat? We delved deeper.
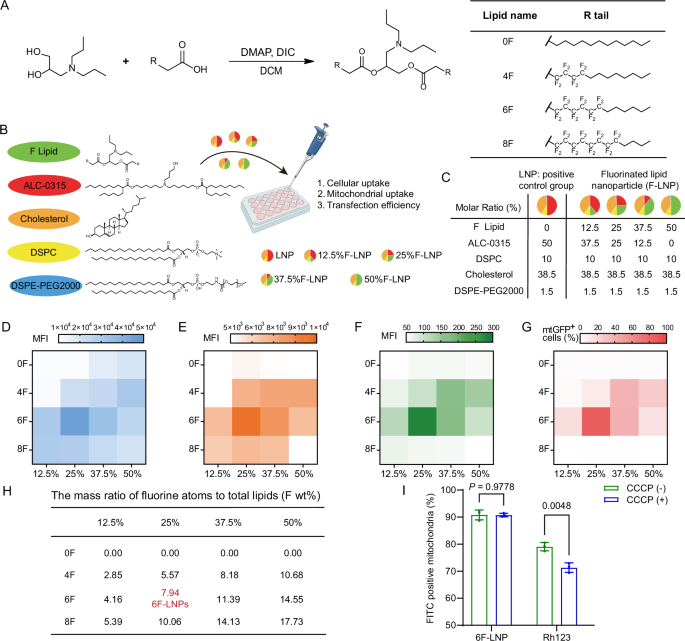
Given the hydrophobic character and lack of positive charge of fluorinated groups, we theorized that F-LNPs would be more likely to interact with hydrophobic mitochondrial structures, such as lipid components, resulting in high mitochondrial affinity. To test this, we conducted a non-targeted lipidomics analysis. The results revealed a strong affinity of F-LNPs for cardiolipin, a unique phospholipid that is almost exclusively found in the inner mitochondrial membrane, facilitating their anchoring to the mitochondrial surface. The fluorinated lipids are thus poised to facilitate membrane penetration due to their unique physicochemical properties.
An important finding was that the fluorine content is critical for delivery efficiency. We identified an optimal fluorination ratio (7.94% of total lipid mass), at which efficiency peaked; deviations from this optimum, whether too high or too low, decreased performance. This finding compelled us to move beyond the simplistic notion that "fluorination is good" and to appreciate the delicate balance required in nanoparticle engineering. Too little fluorine results in a weak membrane-translocating effect, while too much can cause excessive nanoparticle rigidity, alter cardiolipin interactions, or compromise self-assembly. This provides a clear and crucial design principle for advancing this technology.
The application: Delivering hope in disease models
The therapeutic potential was assessed in disease-relevant models, including cells with pathogenic mtDNA mutations and male mouse models of mitochondrial disease. The optimized F-LNPs demonstrated consistently high mitochondrial transfection efficiency under pathological conditions and conferred a significant therapeutic benefit. These results provide crucial confirmation that our platform operates independently of the pathological microenvironment and represents a viable treatment strategy for mitochondrial disorders.
Looking forward: A new platform for mitochondrial medicine
We have provided a robust proof-of-concept for a safe and efficient mitochondrial gene delivery system that discards the traditional dependency on the MMP. We demonstrate that fluorination offers a potentially universal strategy for a mitochondrial delivery platform. The same fundamental principles could be adapted not only for functional supplementation of mutant genes but also for mitochondrial gene silencing and even gene editing. It is our sincere hope that this "fluorine-key" approach will address the long-standing challenge of mitochondrial delivery, transforming the treatment of mitochondrial diseases from a daunting challenge into a tangible goal.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in