Diving into How the Stromal Microenvironment Supports Prostate Cancer Growth and Metastasis
Published in Cancer

Our recent Nature Communications study of stromal cells in prostate cancer has been a multi-year endeavor, informed by an evolving understanding of the tumor microenvironment.
Stromal cells, found in all organs, contribute to wound-healing, blood vessel formation and structural support for tissues. Scientists know that tumors often co-opt stromal cells to create a more supportive molecular environment for tumor growth and survival. Tumor progression is driven not just by the acquisition of new mutations in cancerous cells, but also by tumor-induced—and tumor-supporting—changes in non-cancerous cells in the stroma.
This led to our initial interest in delineating the stromal microenvironment's role, particularly focusing on mesenchymal cells, in prostate cancer progression.
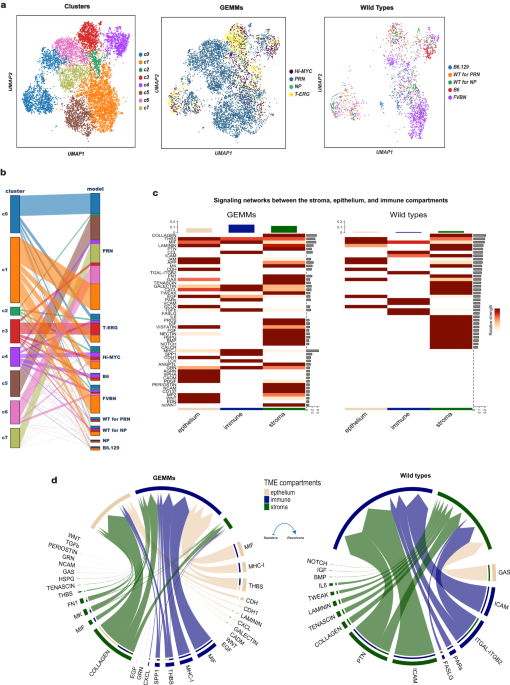
Building upon our previous work, notably the 2017 Nature Communications study by Tyekutcheva et al, my laboratory delved into the diverse gene expression profiles of stromal cells. In the current study, examining prostate tumor mouse models, as well as samples from human patients, we identified eight subpopulations of stromal cells with distinct tumor-associated patterns of gene activity. We found that stromal cells' behavior changes in the presence of a tumor and varies depending on their location within the prostate. Furthermore, we found that certain changes in these patterns predicted metastasis.
The Emerging Importance of Tumor Microenvironment Cells
These findings underscore the stroma's active role in prostate cancer progression, challenging the conventional view of the prostate microenvironment as a passive entity. This comprehensive approach underscored the dynamic and influential nature of stromal cells in the progression of prostate cancer, paving the way for innovative research directions in this field.
In our most recent paper, we combined single-cell RNA sequencing with AI-based analytical methods, to identify eight major subpopulations of tumor-associated stromal cells—in both mouse and human tumors—based on their distinct patterns of gene activity when a tumor is present.
We showed that some of these tumor-associated patterns change when the cells acquire new cancer-driving mutations, and as tumors become metastatic. Strikingly, we found that stromal cells surrounding prostate tumors often foster a molecular environment similar to bone, essentially preparing the tumor cells to spread to bones—a common site for prostate cancer metastasis.
This discovery was indeed surprising and illuminating. While it's known that prostate cancer often metastasizes to the bone, the role of stromal cells in creating a bone-like environment was unexpected. This observation indicates a potential mechanism by which prostate cancer cells prepare for bone metastasis.
The analysis yielded lists of signaling proteins and networks that become abnormally active or inactive during these changes. These signaling interactions between tumor cells, stromal cells and immune cells might be targets for future prostate cancer treatments to block metastasis.
Our study lays the groundwork for a variety of compelling future research paths, each holding the potential to greatly advance our understanding of prostate cancer. One critical direction is the in-depth analysis of the eight distinct subpopulations of stromal cells that we identified, each with its unique pattern of tumor-associated gene activity. Delving into the specific functions and interactions of these subpopulations could shed light on the intricate mechanisms driving prostate cancer progression and metastasis.
This exploration is particularly relevant for unraveling how these stromal cell states might contribute to therapeutic resistance and influence disease progression over time, which could lead to the development of more targeted and effective treatment strategies. Simultaneously, our ongoing work emphasizes the importance of temporal analysis in these cellular populations. We are actively engaged in studies using mouse models such as high-MYC and TRAMP to track the evolution of stromal and immune cells over various cancer stages.
This approach is crucial for understanding how these populations morph and adapt throughout the cancer's lifecycle. Moreover, we are extending our research to include the examination of single-cell profiles from metastatic lesions in the lung and liver. These investigations will provide deeper insights into the metastatic process, particularly in understanding how the tumor microenvironment dynamically interacts and evolves in the context of metastasis.
Our insights with those of other labs suggest targets in the intricate communications within the tumor microenvironment. By disrupting these specific pathways, we aim to inhibit metastasis. We have also identified thrombospondin and macrophage-inhibiting factors as significant players creating an immunosuppressive environment within stromal clusters. These findings highlight pathways that are not just theoretically intriguing but are eminently targetable in a clinical setting.
Our research underscores the vital need to include stromal elements in a more holistic approach to treatment. This expanded focus could revolutionize treatment strategies, not only for prostate cancer but also potentially for other malignancies exhibiting similar stromal dynamics and for broader advances in oncology.
By understanding the role of stromal cells in disease progression and metastasis, we open new pathways for research and the development of innovative treatment strategies. This is particularly relevant in various genitourinary cancers and potentially in other cancer types, highlighting the broad applicability and significance of our work in the field of cancer research and treatment.
# # #
Nature Communications blog post by Dr. Massimo Loda, Chair of the Department of Pathology and Laboratory Medicine at Weill Cornell Medicine, with Dr. Hubert Pakula, and Weill Cornell Medicine writer Jim Schnabel.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in