Drosophila cryptochrome: Balance between light sensitivity and dark recovery
Published in Chemistry
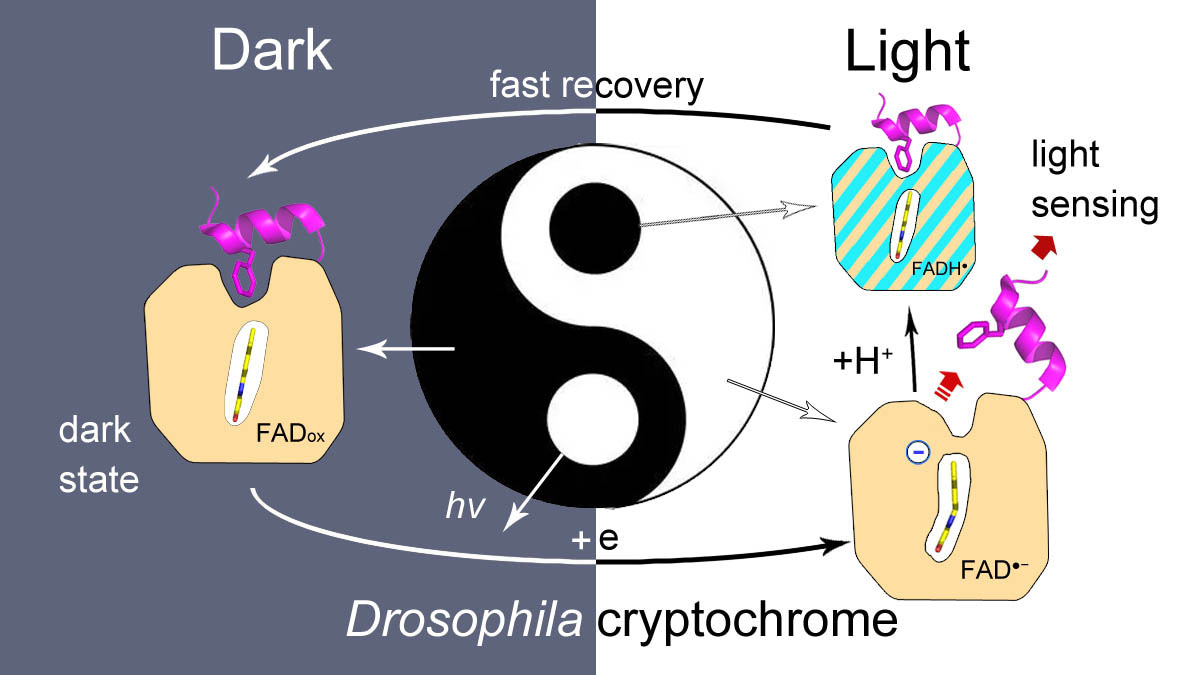
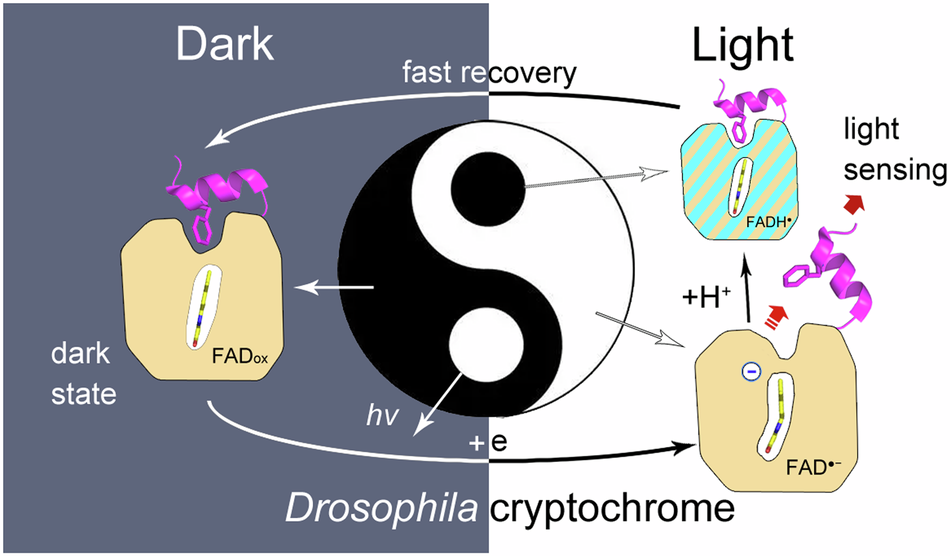
Drosophila cryptochrome (dCry) is an important component of the Drosophila circadian clock system that entrains the circadian rhythm with light cues. dCry contains an FAD cofactor and a C-terminal tail (CTT) (Fig. 1A). The release of CTT upon illumination is a crucial step for the light sensing of dCry. However, the roles of FAD in different ionic and protonation states are not fully understood.
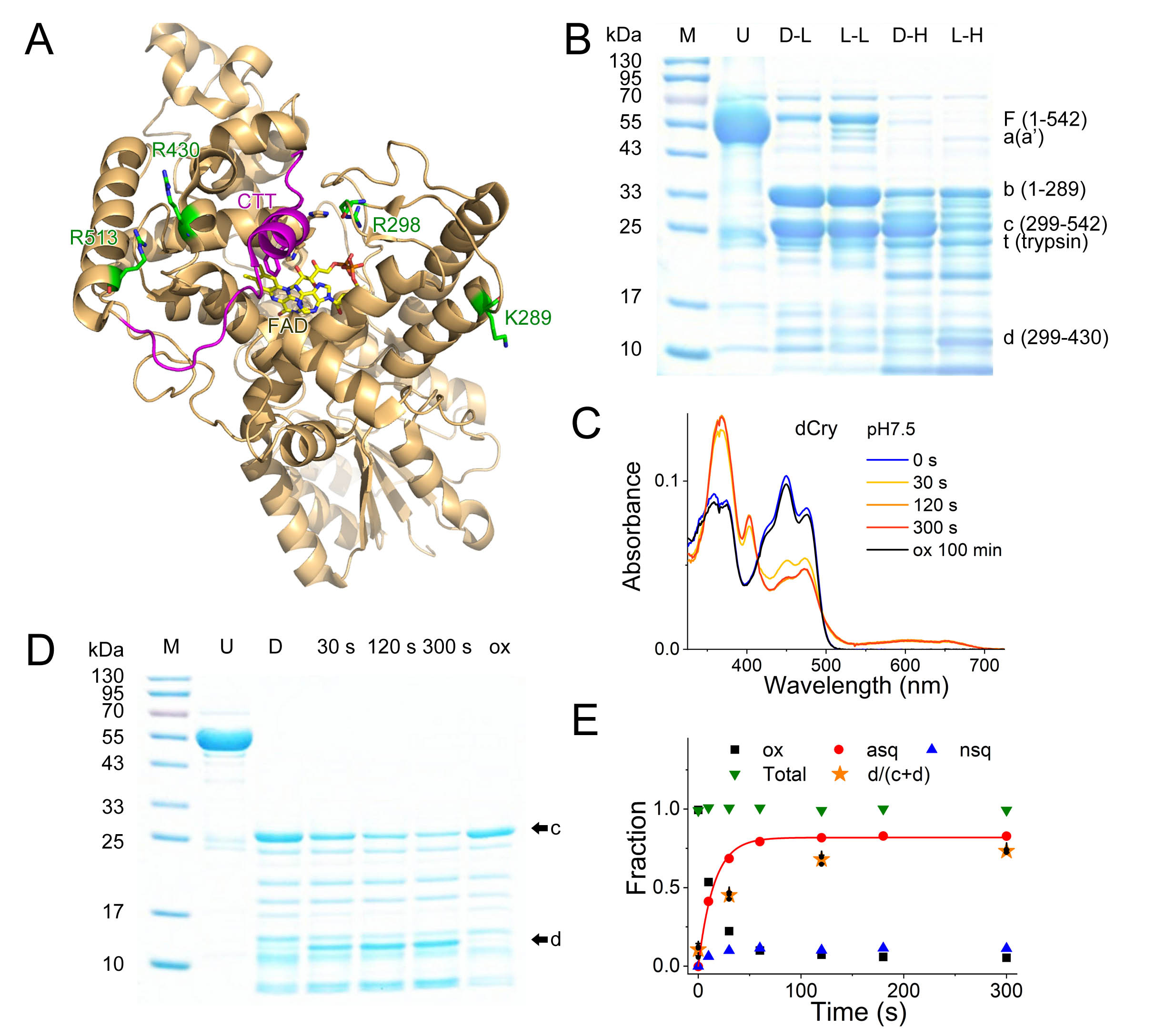
Limited trypsin digestion is a powerful method that has already been used to investigate the light-induced conformational changes of dCry (Ozturk, 2011; Vaidya, 2013). Generally, low concentrations of trypsin or short incubation period may aid in detecting subtle conformational changes. However, in practice, these protocols are difficult to perform, and false-positive or false-negative results are often obtained. This was due to the strong susceptibilities of Lys289 and Arg298 under both dark and light conditions, as most full-length dCry was cleaved at these positions to form fragments b (1-289) and c (299-542) before the formation of light-induced fragments (a, 1-503, and a’, 1-513), making these fragments too faint to be easily detected (Fig. 1B, lanes D-L and L-L).
My student Wenlong Xie used a different strategy. He used a high concentration of trypsin (1:1 molar ratio) with a relatively long incubation time (1 min). Under these conditions, secondary cleavage of fragment c at Arg430 was observed upon light exposure, resulting in an ~15 kDa fragment d (299-430) (Fig. 1B, lanes D-H and L-H). This protocol is very robust and highly reproducible. The intensity ratios of fragment d to the sum of fragments c and d corresponded well with anionic semiquinone (asq) state formation during photoreduction, which proved that CTT release was triggered by asq formation (Fig. 1C, 1D and 1E).
By using this protocol, it was demonstrated that neutral semiquinone (nsq)-forming mutants (C416N and L405E/C416N) barely released CTT under the nsq state, which was in line with a previous report (Chandrasekaran, 2021). Nevertheless, we found that long-term illumination of the nsq-forming mutants produced some anionic hydroquinone (hq) state, which also triggered CTT release.
Wenlong also investigated the pH dependence of CTT release of wild-type dCry during photoreduction. He provided a result that CTT release was inhibited under acidic conditions, accompanied by more nsq formation; and was enhanced under basic conditions when asq was formed predominantly. First, I did not believe this result because I have read several literatures declared that protonation at a key site His378 was important for the activation of dCry and that higher pH inhibited signaling-state formation (Ganguly, 2016; Berntsson, 2019; Einholz, 2021). However, he insisted on his result and repeated the experiments several times. Finally, we confirmed that the result was correct. These findings unified the conclusion from the nsq-forming mutants that nsq formation inhibited CTT release due to the lack of a negative charge and that asq formation promoted CTT release. The formation of a proper fraction of nsq in dCry under neutral conditions moderately suppressed CTT release but increased the oxidation rates of the total semiquinone, resulting in faster recovery when dCry was returned to the dark.
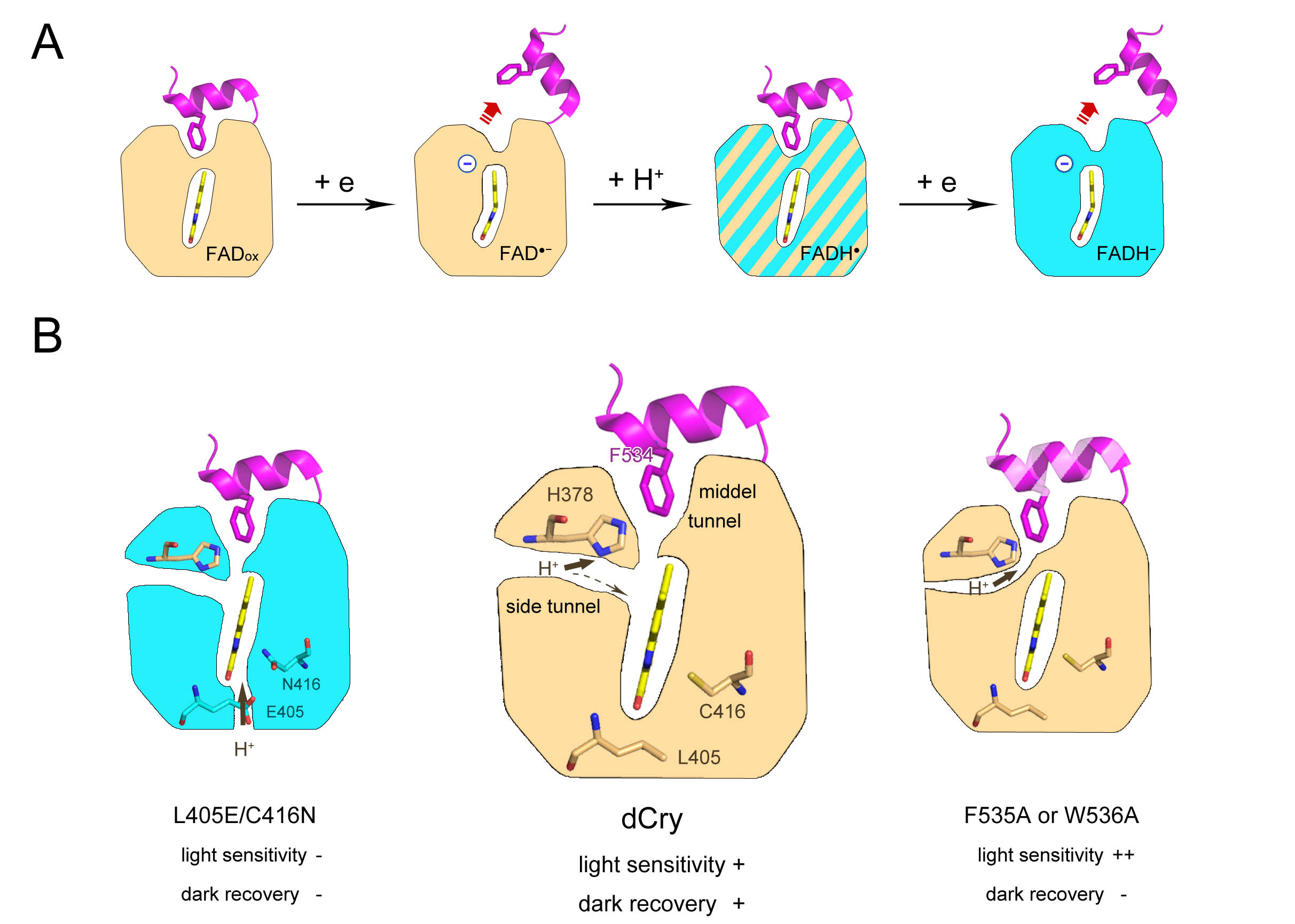
The mutants of dCry in CTT, including the one deleted CTT (dCT, 1-516), and F534A, F535A, and W536A were investigated. It was curious that all these mutants produced nearly pure asq state during photoreduction. Nevertheless, the F535A and W536A mutants exhibited enhanced CTT release. The enhanced light-sensing ability of W536A was in line with the previous work, in which a W536 mutant was used in kinetic experiments because it produced sharper light/dark band differences in the trypsin proteolysis assay (Vaidya, 2013). These phenomena could be easily explained by the fact that the asq fractions are predominant in the CTT mutants during photoreduction, which is more competent to induce CTT release. Dr. Xiuxiu Wang performed molecular dynamics simulations of these mutants. It was revealed that α8 in all of the mutants in CTT shifted inward, making the entrances of a side tunnel narrower. Nevertheless, the curvature of the tunnel of the CTT mutants was lower than that of wild-type dCry. The protons might leak to the solution through the less curved tunnel, which would also prevent the protonation of FAD. However, these mutants could not recover quickly to the dark state due to their lower oxidation rates. This might be the reason that natural selection did not favor these mutants as photoreceptors.
Another student of mine, Mengqi Wan, and his teammates performed many experiments on His378 mutants. They showed that the nsq fractions of the initially photoreduced H378A and H378K mutants were even greater than those of wild-type dCry. However, the H378D mutant formed a nearly pure asq state during photoreduction. The methyl side chain of the alanine residue cannot accept or provide protons. The side chain of the lysine residue is already protonated under neutral conditions. These two residues had little effect on proton transfer through the side tunnel to FAD. Therefore, higher fractions of nsq formed during photoreduction of the H378A and H378K mutants. The carboxyl side chain of aspartic acid and the imidazole side chain of histidine may attract protons before being transferred to FAD and suppress the protonation of large amounts of asq in the H378D mutant and wild-type dCry. These results revealed that the residue at site 378 could adjust the protonation degree of FAD during photoreduction.
In summary, we demonstrated that the negative charge on FAD is crucial for CTT release in this work. The side tunnel was found in dCry, through which protons could be transferred to a fraction of asq, protonating it into nsq. Proper fractions of nsq formed in dCry are important for achieving a balance between light sensitivity and dark recovery (Fig. 2).
Follow the Topic
-
Communications Chemistry

An open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of the chemical sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
f-block chemistry
Publishing Model: Open Access
Deadline: Feb 28, 2026
Experimental and computational methodology in structural biology
Publishing Model: Open Access
Deadline: Apr 30, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in