Dynamic stretching beyond electron transfer in a homointerpenetrated MOF for enhanced Fenton-like reactions
Published in Earth & Environment
Recently, emerging refractory organic pollutants especially antibiotics in aquatic environment have posed chronic and cumulative risks to ecosystems and human health due to their persistence, bioaccumulation, intrinsic toxicity and their poor biodegradability, further leading to persistent environmental hazards including the spread of antibiotic resistance genes. Therefore, it was essential to seek a suitable technology for the efficient degradation and detoxification of pollutants, thereby reducing the damage to human health and environment. Fenton-like reaction induced by peroxydisulfate (PDS) exhibits exceptional oxidation performance, broad pH applicability and high stability, rendering them irreplaceable for antibiotic degradation and detoxification. Moreover, the degradation and detoxification performance toward emerging refractory organic pollutants crucially depends crucially on the formation of reactive oxygen species (ROSs), including radicals (SO4•−, •OH, and O2•−) and nonradicals (1O2, high-valent metal, and electron transfer process) for PDS activation. One of the critical focuses in Fenton-like reactions is the rational design of heterogeneous catalysts with highly active sites. Our research group from the Beijing University of Civil Engineering and Architecture, Peking University Shenzhen Graduate School and Peking University focuses on the rational development of diverse environmental functional materials, including metal-organic frameworks (MOFs) and their derivatives, metal-based catalysts, titanate materials, and carbon materials as well as their tailored applications in water purification.
https://communities.springernature.com/manage/videos/304182
Typically, it was generally believed that the generation of ROSs, which played a significant role in pollutant degradation process, was primarily realized by controlling the electron transfer efficiency between highly catalytic sites and oxidants. Thus, extensive research had been dedicated to enhancing electron transfer efficiency by designing heterogeneous catalysts like single-atom catalyst via electronic structure regulation. However, due to the complexity of interfacial reaction dynamics in Fenton-like reactions, the dynamic change of catalyst, a critical phenomenon, was often overlooked. It was believed that the dynamic change in catalyst also may played important role in Fenton-like reaction process, which had demonstrated by recent study of dynamic stretching of metal‒oxygen (M‒O) bonds. Not all conventional catalysts exhibited the dynamic stretching effect via chemical bond formation, which poses a significant limitation in Fenton-like reactions. Notably, interpenetrated metal‒organic frameworks (MOFs) consist of two identical networks that were entangled yet non-covalently bonded, exhibiting inherent dynamic flexibility due to the tunable spatial separation between constituent frameworks. Therefore, the large-scale dynamic stretching of interpenetrated MOFs made them ideal model systems for studying the relationship between the dynamic stretching of whole-framework of catalysts and the resulting catalytic activity in Fenton-like reaction.
To verify the above-mentioned discussion, we prepared a homointerpenetrated MOF (denoted BUC-95, CCDC: 2352385) with a flexible dynamic stretching framework and applied for the efficient degradation of various organic pollutants through PDS activation. To deeply explore the relationship between dynamic stretching effect and catalytic performance, another homointerpenetrated MOF (denoted BUC-96) with restricted dynamic stretching effect induced by hydrogen-bonding interactions as an isostructural analogue for comparison. BUC-95 exhibited exceptional degradation efficiency toward diverse electron-rich organic pollutants through a high-valent iron-oxo (Fe(IV)=O)-dominated oxidation pathway. Experimental characterizations and density functional theory (DFT) calculations demonstrated that the adsorption of -OH on BUC-95 not only regulated the d-orbitals electron density of Fe but also greatly reduced the activation energy barrier, ultimately facilitating the formation of Fe(IV)=O and sulfate ions through unique flexible dynamic stretching effect in the BUC-95-OH/PDS system. More importantly, this work strongly demonstrated that the flexible dynamic stretching plays a role beyond electron transfer during PDS activation process.
Electrochemical characterizations confirmed that BUC-96 exhibited stronger electron transfer and donating capabilities during PDS activation. Nevertheless, BUC-96 did not exhibit satisfactory ofloxacin (OFC) degradation performance for PDS activation even with its superior electron transfer ability. Unlike BUC-96, whose framework stretching is restricted by hydrogen bonding, the flexible dynamic stretching in BUC-95 demonstrated by different in situ characterizations (e.g., PXRD, Raman, FTIR, XPS and XAFS) and in situ molecular dynamics simulations facilitates electron transfer from Fe-Fe' sites to PDS, thereby significantly enhancing the OFC degradation. In addition, the developed BUC-95@sponge (BS) achieved >99.99% OFC (10.0 mg L-1) degradation efficiency within 110.0 h and superior detoxification performance. In all, this work offered insights for the rational design and development of MOF catalysts with flexible dynamic stretching structures for sustainable water purification via Fenton-like reactions.
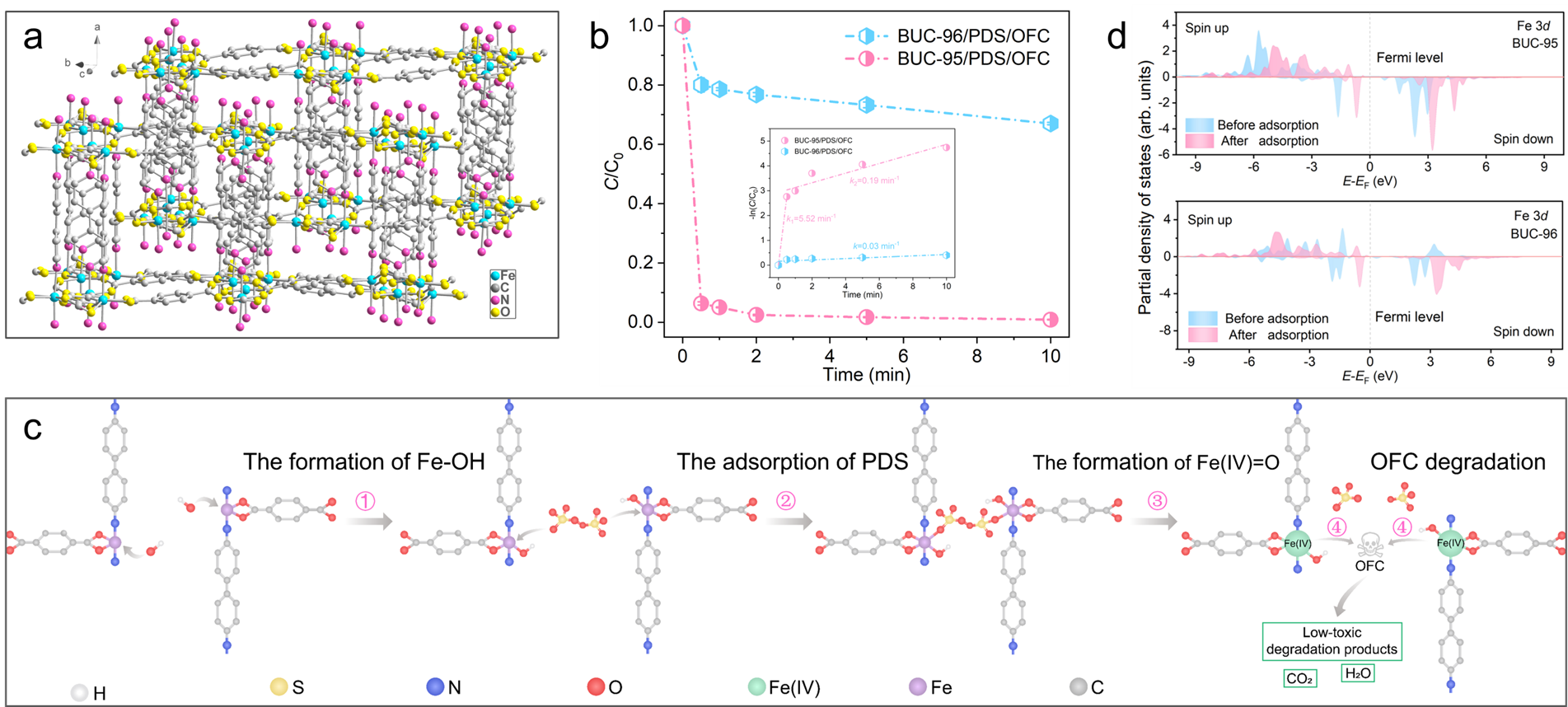
Figure: (a) Flexible dynamic stretching in the homointerpenetrated BUC-95 structure. (b) OFC removal in different reaction systems. (c) The schematic diagram of Fe(IV)=O generation and OFC degradation mechanisms in BUC-95/PDS system. (d) PDOS of Fe 3d before and after PDS adsorption for BUC-95 and BUC-96. Image author: Prof. Dr. Chong-Chen Wang
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in