Electro-assisted Printing of Soft Hydrogels via Controlled Electrochemical Reactions
Published in Materials
Soft hydrogels are the ideal biomaterial for interfacing soft/living tissue due to its similar mechanical properties. Furthermore, hybrid materials with different properties enables the design of smart devices for a wide range of applications, such as controlled drug delivery, tissue engineering, soft actuators, among others. For such smart device, an alternative is to have it controlled electronically using a conductive electrodes (or electrode array), highly desired for bioelectronics. However, the design of a good adherent soft hydrogel (composed of ~90% water) is still quite challenging.
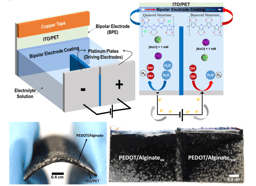
Herein, we developed an easy modification to a 3D-printer nozzle enabling the 3-electrodes configuration to be used in any 3D-printer. It transforms a simple 3D-printer nozzle in a reference and counter electrodes, while using any conductive substrate as working electrode. To demonstrate the versatility of our developed approach, Figure 1 summarizes the whole procedure.
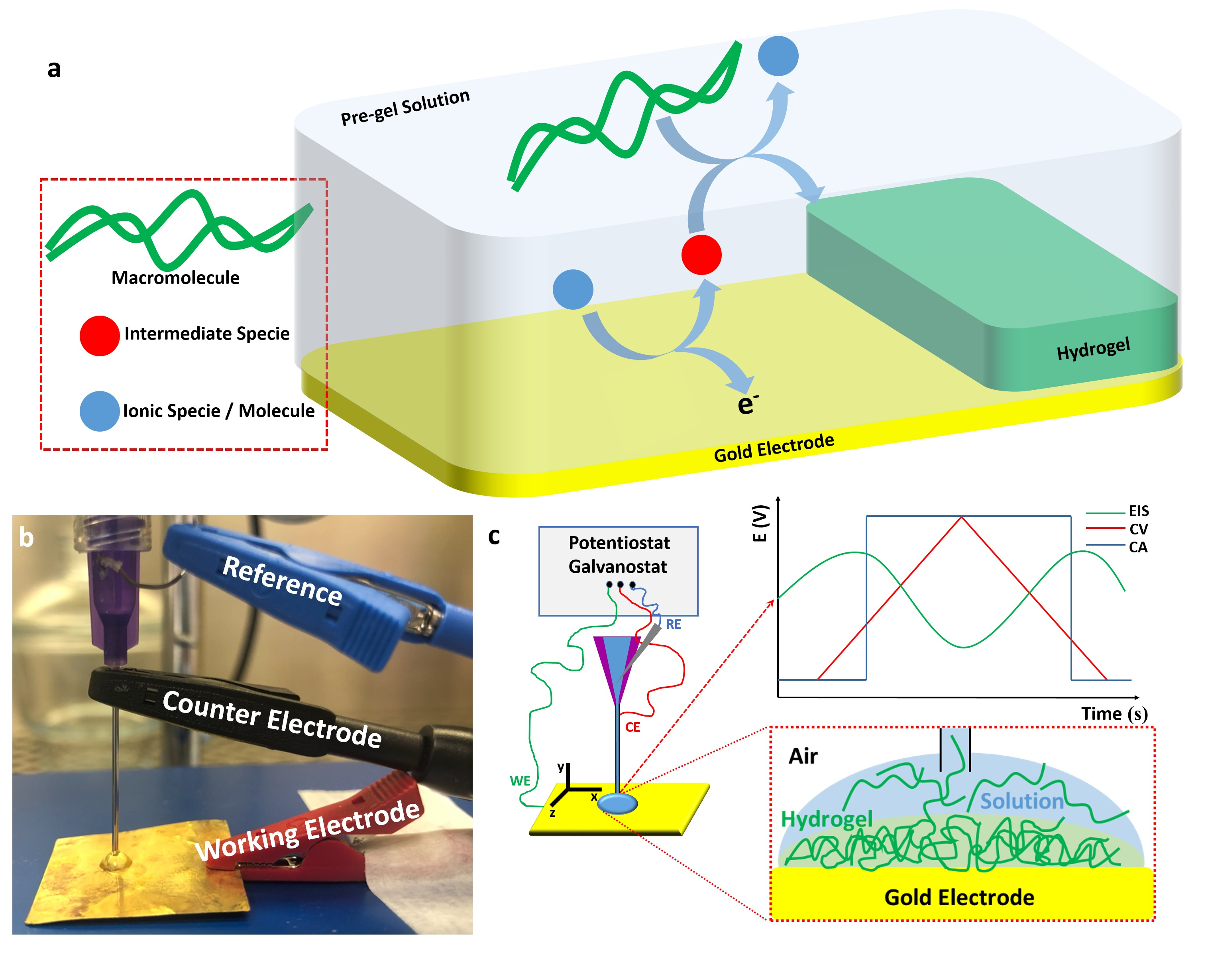
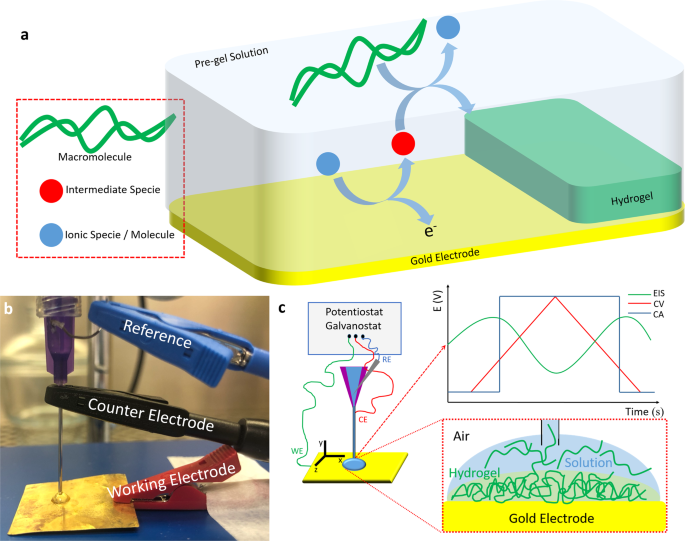
We developed a method by using electrochemical-chemical-chemical (ECC) mechanism is possible to obtain different types of soft hydrogels. Figure 1a shows a schematic representation where an ionic specie or molecule first react at the electrode interface, generating an intermediate specie. Afterwards, it react with the macromolecule promoting the electrodeposition of the soft hydrogel at the electrode's interface. Figure 1b shows a picture of the adaptation we made to the 3D-printer nozzle. We have added a silver wire coated with silver chloride (Ag/AgCl) pseudoreference to the plastic container of the nozzle. We used the stainless steel needle as a counter electrode and connected any conductive surface for the working electrode. When connected all the electrodes to a portable potentiostat/galvonstat, it enabled us to promote any electrochemical experiment in situ (Figure 1c), including the electro-assisted generation of soft hydrogels.
To demonstrate the versatility of our approach, we have used 1) covalently crosslinked chitosan hydrogel; 2) ionically crosslinked alginate hydrogel and 3) hybrid PEDOT/alginate conductive hydrogel.
For the covalently crosslinked chitosan hydrogel: when applying the correct electric potential in presence of chloride ions it is possible to promote the covalently crosslinking of chitosan over gold surface. We have tested low and high molecular weight chitosan for the same reaction. A linear relationship between the total charge density applied and the thickness of the hydrogel produced demonstrates the electronical control over the soft hydrogel production.
For the ionically crosslinked alginate hydrogel: when electric potential ≥ +3.0 V (vs. Ag/AgCl pseudoreference) is applied, it promotes the water hydrolysis reaction triggering the ionically crosslinking alginate in presence of calcium carbonate particles. Again, linear relationship between the total charge density applied and the thickness of the hydrogel demonstrates the fine electronical control over the soft hydrogel generation.
For the hybrid PEDOT/alginate conductive hydrogel: we promoted the electrodeposition of the conducting polymer PEDOT doped with alginate. This reaction shown a slower kinetics of growing due to diffusional limited reaction of EDOT polymerization. The electrochemical characterization was made in situ. The addition of PEDOT clearly improved the electronical properties of the hybrid hydrogel, with reduced resistance and increased capacitance.
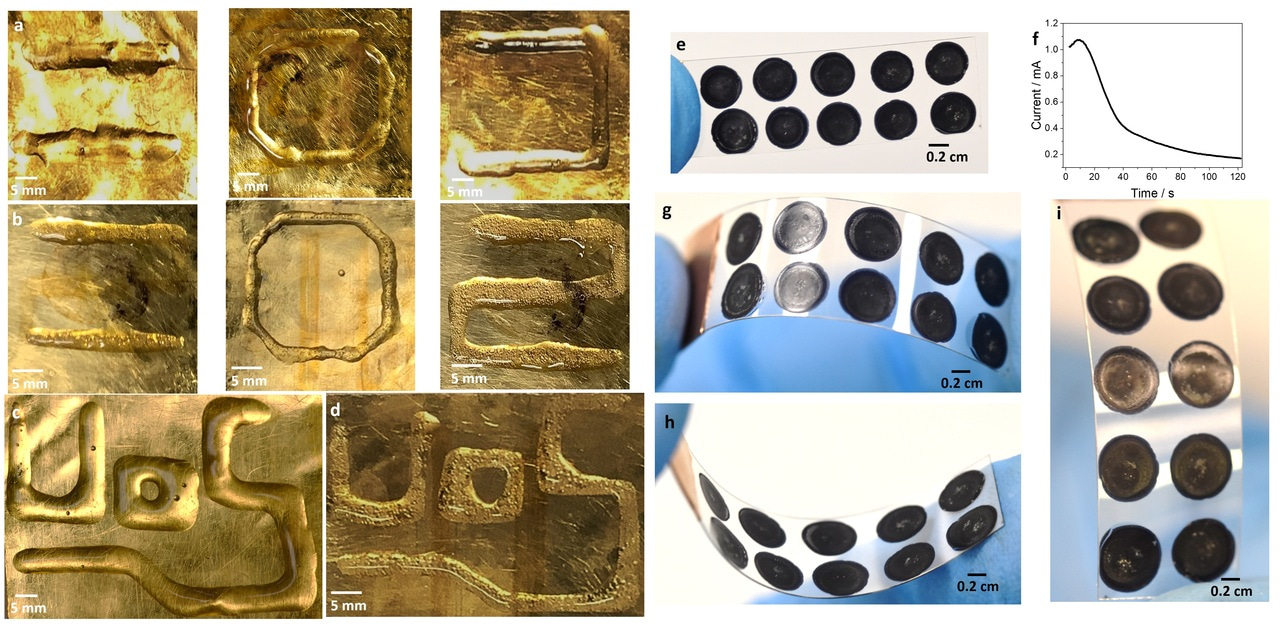
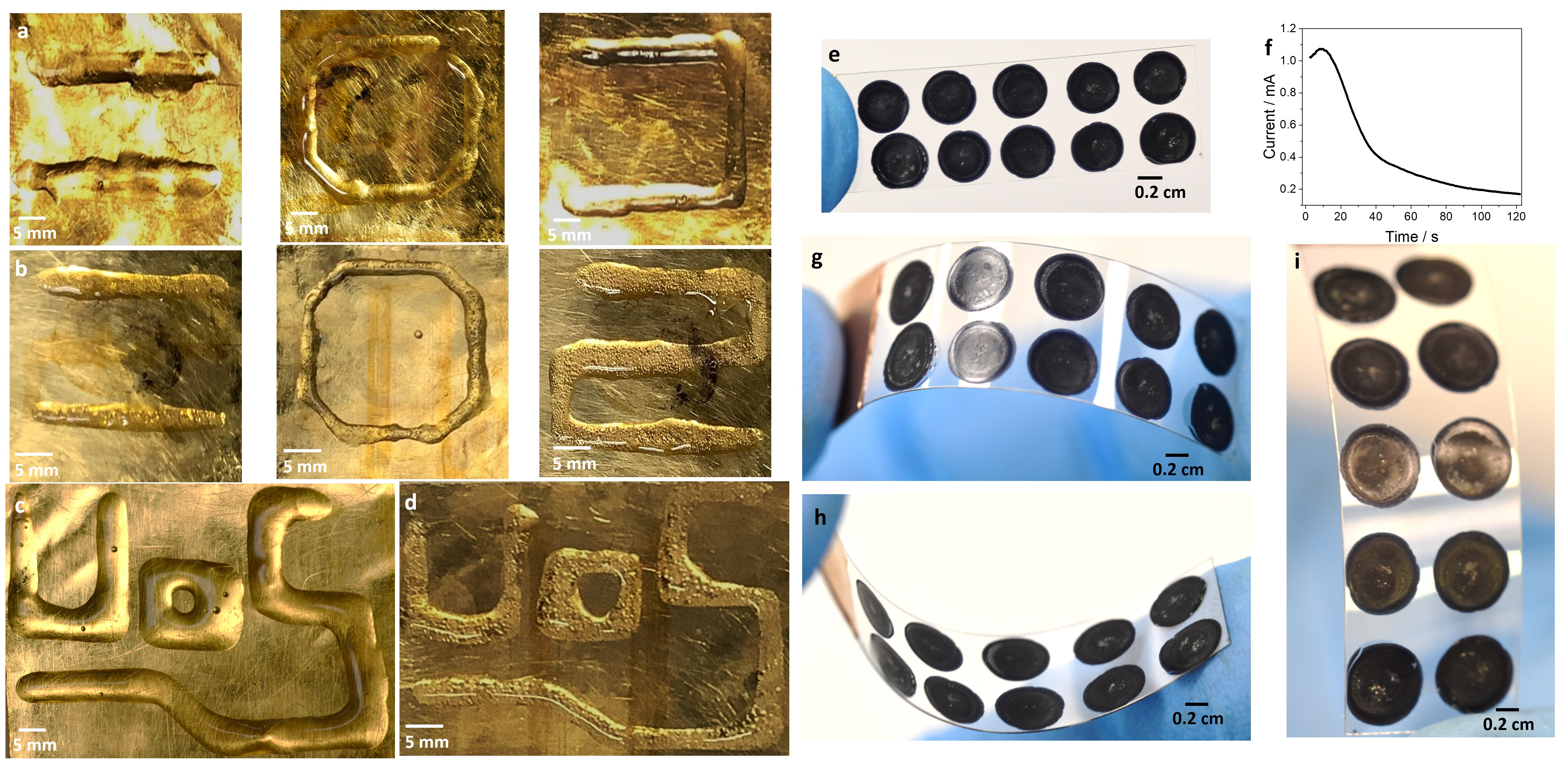
Last but not least, we have coupled the portable potentiostat to a 3D-printer and patterned all the previously discussed hydrogels in different shapes (Figure 2).
For further information, please read our published article in Nature Communications, Electro-assisted printing of soft hydrogels via controlled electrochemical reactions | Nature Communications
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026
Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in